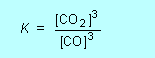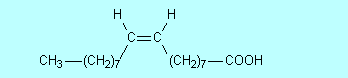|
Two major considerations for teachers In selecting the one option (of the five available) for their students to study, are first their students' likely interest in the topic and secondly their ability to cope with the amount and complexity of the material. Other considerations are likely to be how well the material integrates with and reinforces core material and how straight-forward and/or predictable are likely examination questions on the material. The availability of equipment for laboratory work is another consideration. Judging by the number of pages I needed to cover the material in each of the three options treated in Conquering Chemistry, the Shipwrecks, Corrosion and Conservation option appears to be the shortest, and the material does to a significant extent reinforce and extend core material, though some of it can be difficult to understand for weaker students. In some ways the material in Industrial chemistry may be more straight forward, but apart from chemical equilibrium it does not flow as directly from core material. There appears to be much more material in Forensic chemistry and much of it is quite complex for students whose background in organic chemistry is just the core material. While a good option for students with a strong biological interest, it could be daunting for weaker students. With the current heavy emphasis on factual recall in exam questions, the quantity of material to be assimilated is probably a key consideration in selecting an option. In writing Conquering Chemistry I thought that the other two options, Biochemistry of movement and Chemistry of art, contained too much material of too diverse a nature for the seven weeks available for the study of the option. Of course these options will particularly appeal to certain groups of students those strongly interested in sport and dance and those with a strong artistic bent and strong motivation can easily overcome barriers that may seem daunting to others.
HSC exam questions in the Options section of the paper differ somewhat from questions in the core section in that .
Because of this absence of calculations and
problems from the options sections of the HSC exam paper, there will be no
Further Exercises sections on the Options pages (except for a few
interspersed through the text). 2. Replacement of natural products When I was writing CCHSC it never occurred
to me that the one dot point in Syllabus Section 9.5.1 would ever be the subject
of a seven mark question and hence the succinct one-paragraph account of each of
rubber, soap and wool on pages 313-4. However it sure happened in 2006. The
examiners gave a sample answer based on rubber in their Examiners' Report for
2006 (see BOS website) something they have very rarely, if ever, done
before, and that answer included more polymer chemistry than was required in
Module 1, While the one paragraph on page 314 may appear to answer the question
asked, it is not worth seven marks, so when you get a question like this, you
need to pad out your answer with chemistry, even if it is only vaguely related.
In my answer ( ) I have included structures of a soap anion and a synthetic
detergent anion, and to be on the safe side included the equation for
saponification of a fat to form soap. Since you have 12 minutes for this answer
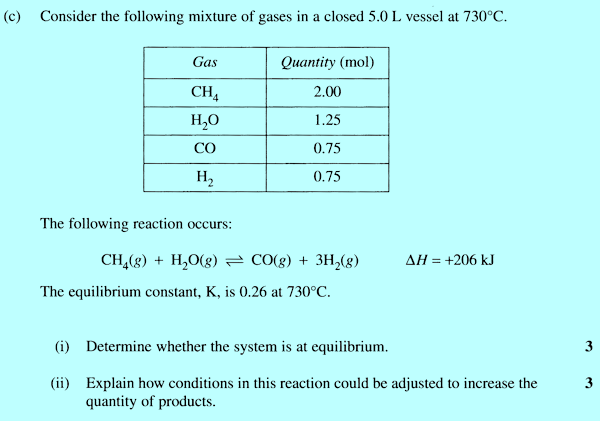
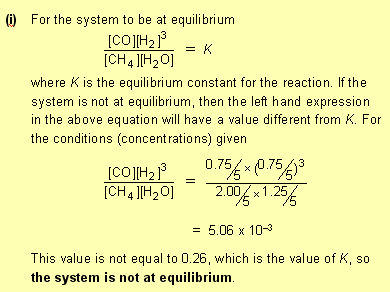
that is not wasting time, just making sure of the marks. In the treatment of equilibrium constants in CCHSC only homogeneous gas phase reactions are discussed. However in the 2007 HSC exam paper a heterogeneous reaction (one involving a solid as well as gases) was used. There is an extra 'rule' for writing equilibrium expressions to those given on page 321, namely: When a pure solid is involved in an equilibrium reaction, there is no term for the solid in the equilibrium expression. For example for the reaction that occurs in a blast furnace for extracting iron from its ore: Fe2O3(s) + 3CO(g)
Exercises 4. Modelling an equilibrium reaction One of the 'first hand experiences' required for this Option is to model an equilibrium reaction. The example given in Exercise 5 on page 317 of CCHSC is fine on paper. However it may be a little difficult to set up in practice in that it requires the hole B in the container A to be able to produce a flow rate (at an achievable height of water in the container) that matches the pumping speed of the pump (a small one for an indoor water feature?). An alternative experiment is described in www.chymist.com/equilibrium.pdf
(as of 14/2/2009 you do not appear to need name and password; just leave blank
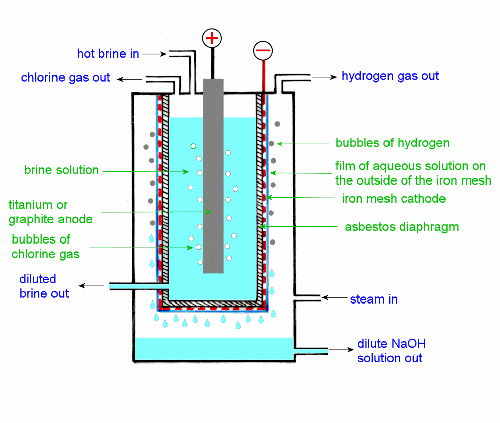
and click OK.) 5. Manufacture of sodium hydroxide The diaphragm cell was the apparatus used in one of the two early methods of manufacturing sodium hydroxide industrially. Very few plants using this technology are still in use. This cell is included in the HSC to help show how advances in science lead to developments of new and better technology. The diagram of this cell in CCHSC, Figure 10.4 on page 353 is a schematic one, drawn to look like an ordinary hydrolysis setup that students are familiar with. Figure 1 is a diagram that more closely resembles what these cells actually looked like. There is an iron mesh container, the cathode, with an asbestos diaphragm lining its insides; in this is placed hot brine solution and the anode which in earlier times was graphite though later on titanium was preferred. This container is suspended inside a larger container. Steam is blown through this to keep the outside of the mesh and diaphragm moist (and to keep the whole cell warm and so speed up the process). On the ion mesh cathode water is reduced to hydrogen gas and hydroxide. Water and sodium ions diffuse through the asbestos diaphragm (along with a small amount of chloride) and sodium hydroxide solution drips off the bottom of the cathode. The chemistry and ion migration is exactly as shown in Figure 10.4. The difference between this old diaphragm cell and the currently used membrane cell is shown in Figure 10.7 on page 356.
Figure 1 The diaphragm cell 6. Diaphragm cell ® mercury cell ® membrane cell? CCHSC claims that the diaphragm cell was the first to be used (p 352) and that the mercury cell was developed to overcome its disadvantages (p 354). The 2007 HSC exam paper implies that the mercury cell was first (and I guess the syllabus document (Section 9.5.4) is implying the same thing). The website on page 357 also says the mercury cell came first. The claim that the diaphragm cell was first is technically true, but my claim that the mercury cell was developed later and in response to problems with the diaphragm cell is not correct. A history of the chlor-alkali industry is
presented in the introduction of Stringer, Ruth and Johnston, Paul, Chlorine
and the Environment, an overview of the chlorine industry, Springer, 2001.
The first commercial diaphragm cell was used in Frankfurt in 1891, followed by
an improved (continuous flow) version (in the US) in 1893. The mercury cell was
developed independently of this and the first commercial plant using it was
opened in 1897 (in the UK). Both technologies developed and expanded more or
less in parallel and independently of each other. Various factors determined which type of cell would be used as
different plants were established. Both types had disadvantages (see CCHSC)
and different cost considerations. Slowly, despite its higher construction
costs, the mercury cell overtook the
diaphragm cell in popularity. The problems of contamination of product with
chloride and escape of dangerous asbestos were recognised long before it was
realised that discharge of small amounts of elemental mercury into oceans was
harmful. It was not until the 1960s that it was realised that certain bacteria
converted insoluble mercury into soluble mercury dimethyl Hg(CH3)2
that could be absorbed by other organisms and ultimately by humans with serious
detrimental effects. Governments put more and more stringent
restrictions on the amount of mercury these plants could discharge and the
plants spent more and more money trying to meet them. Pressure was on to develop
an alternative cell. There was no going back to an asbestos diaphragm because
the health dangers of asbestos had been well recognised by the 1970s.
Eventually the membrane cell was developed and now all new plants are of this
type, though many mercury cell (and a few diaphragm cell) plants are still in
operation. That makes answering the very similar questions in the 2005 and 2007 HSC exam
papers a bit awkward. I
have now revised my original answer to the 2005 question in the Answers to
HSC exam papers section of the website (go back to CCHSC opening page
to get there) and have offered an answer to the 2007 question below. What do you do if the HSC examiners ask you to
define saponification? Give the correct chemical definition as given in CCHSC
on page 358 or give the less correct definition in the syllabus document Section
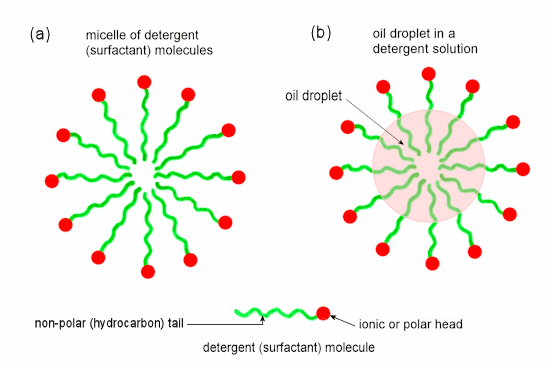
9.5.5? The safest procedure would be to give both as in: CCHSC unfortunately does not describe the nature of detergent (surfactant) solutions very well. All detergent molecules have a long hydrocarbon tail that is not soluble in water. When such compounds are placed in water, these hydrocarbon tails stick to one another and leave the ionic or polar end of the molecules pointing outwards and into the water which is strongly attracted to these heads; this forms what is called a micelle as shown in Figure 2(a). Micelles are three dimensional and typically contain 50 to 100 molecules. A 'solution' of a detergent is not strictly a solution at all; it is a dispersion of these small micelles throughout the water. The presence of these micelles instead of just individual molecules dispersed through the water causes detergent solutions to look cloudy. We say that the water attracting head is hydrophilic (water loving) while the water repelling tail is hydrophobic (water hating).
Figure 2 (a) a micelle of detergent molecules in water (b) the way oil or grease gets dispersed through a detergent solution to form an emulsion When oil is added to such a solution, the hydrocarbon tails 'grab' the oil and dissolve in it and so we get the effect shown in Figure 2(b) and in the right hand drawing of Figure 10.11 (page 365). This disperses the oil through the water or solubilises the dirt as discussed in Figure 10.11.This forms an emulsion.
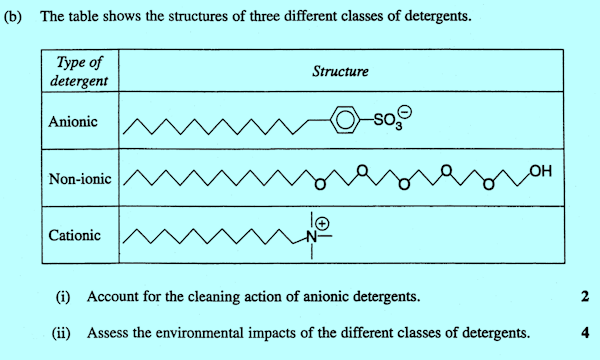
9. Detergents and environmental effects CCHSC (page 364-5) explains that the word detergent can have two meanings, one for the mixture that people commonly buy as a detergent such as laundry detergent, washing-up liquid or dishwashing powder, while the second is for the actual surfactant, the compound that reduces the surface tension of the water and so sets off the cleaning process. Under the present syllabus HSC examiners have never used the term surfactant and in the contexts in which they have used detergent it has generally been clear that they mean the surfactant compound. However on several occasions there has been a question about the environmental effects of detergents. The problem with this is that today surfactants have an almost negligible effect on the environment. Anionic and non-ionic surfactants biodegrade quite rapidly perhaps not quite as fast as soap, but quickly enough and they are easily destroyed by good sewage treatment. Cationic detergents can have a small detrimental effect on the environment in that in large concentrations they can kill bacteria before the bacteria can destroy them. Hence some care is needed to avoid excessive use of them. In the 1960s there was a problem with anionic surfactants (the only type available then), because the original anionic surfactants were branched chain molecules that did not easily biodegrade and so there were often build-ups of them in waterways with detrimental effects on aquatic life forms and unsightly foam on many streams and lakes. However that problem disappeared in the 1970s when the current linear anionic surfactants came into use. Today the environmental harm caused by detergents comes from another ingredient in the detergent mixtures that are sold, not from the surfactant. Phosphate is added to detergent mixtures to help keep the dirt released during washing in suspension and to stop it settling back on the clothes etc. It is called a builder. This phosphate in these detergent mixtures is of considerable environmental concern because as explained in CCHSC pages 286-8 and 370 it can lead to excessive algal growth. If there are only one or two marks for the environmental effects part of the question, then mention of the toxicity of cationics is probably enough but if there are three or four marks, you probably need to talk about phosphates as well.
It is possible to draw a great variety of flow charts for the Solvay process for making sodium carbonate; two are given in CCHSC on pages 372 and 384. Do not get 'thrown' by seeing a diagram different from what you are used to: just follow it through and see that it works. The essential features of the Solvay process are (a) a brine solution is saturated with ammonia and carbon dioxide and this produces a precipitate of sodium hydrogen carbonate (b) sodium hydrogen carbonate is heated to form sodium carbonate (c) the reactant carbon dioxide comes from heating calcium carbonate (limestone) and (d) ammonia is recycled (by reacting product ammonium chloride with calcium hydroxide formed from calcium oxide, the other product of heating limestone. Environmental effects are often included with a question on the Solvay process. The main ones are (a) disposal of the waste product calcium chloride (b) damage caused by extracting and transporting the reactants (limestone and solid sodium chloride or brine solution) and (c) air pollution from the inevitable escape of small quantities of ammonia. The usual effects of heavy industry such as dust and noise are also involved. Choice of a suitable location for such a plant
has sometimes figures in HSC questions on the Solvay process. Location near the
ocean (for easy disposal of calcium chloride solution) is always a high
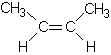
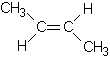
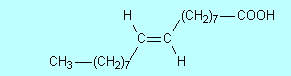
priority. Supplementary material We hear a lot today about avoiding trans fats; they are considered worse for our health than saturated fats, so what are they? To answer this we need to explain what is called cis-trans isomerism. In CCPC page 263-5 alkenes were introduced. It was shown there and in Table 9.5 on page 255 that the geometry around a double bond is planar; around each C atom of the double bond the bond angles are 120o. This means that for 2-butene there are two possible isomers:
In (1) both methyl groups
are on the same side of the double bond while in (2) they are on opposite sides.
When the two groups are on the same side of the double bond, we call the
molecule a cis isomer and when they are on opposite sides we call it a trans
isomer. So (1) is cis-2-butene while (2) is trans-2-butene. Figure
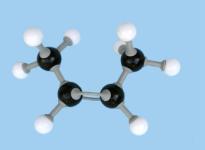
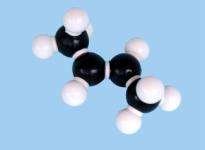
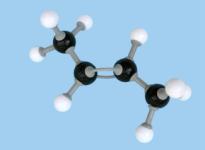
3 shows molecular models of these compounds. These two structures represent quite distinct
compounds; they have different melting and boiling points and densities and have
slightly different chemical reactivities.
Figure 3 Space-filling and ball-and-stick models of cis- and trans-2-butene Double bonds occur in oleic, linoleic and linolenic acids (Table 10.3 on page 361 of CCHSC) and in other naturally occurring fatty acids. In all of these the arrangement around the double bond is cis; for oleic acid:.
Unfortunately in the process of hydrogenating only some of the double bonds, some of the remaining ones get converted from cis into trans. So cooking products made in this way can end up containing significant amounts of trans fats. Oleic acid gives rise to the trans compound, elaidic acid:
Omega-3 fatty acids are an essential part of the human diet, but what are they? They are unsaturated fatty acids with one double bond starting on the third carbon atom from the hydrocarbon end of the molecule and running to the fourth. Omega is used to denote the end of the acid molecule that is not the carboxylic acid end. Linolenic acid (Table 10.3) is an omega-3 fatty acid. Another one is DHA, docosahexaenoic acid; this is a C22 molecule with six double bonds, one of which starts from the third carbon atom from the omega end (the others start from the 6th, 9th, 12th, 15th and 18th carbons from that end; try drawing the structure). Some omega-3 fatty acids are called essential fatty acids, meaning that they are essential to the human metabolism. Unfortunately our bodies cannot make them so we must include them in our diet. A good source of these acids is oily fish tuna, salmon, cod, etc.
Answers to selected HSC exam questions 2004
Question 28(b) (Copied from Board of Studies website)
2007 Question 28(b) Over the past century the production of sodium hydroxide has evolved from the mercury process, to the diaphragm process, to the membrane process. Analyse
the factors that contributed to each of the changes in the production
process. (Transcribed from the Board of Studies website)
2007 Question 28(e)
2008 Question 29(c)
(Copied from Board of Studies website)
Answers
to the 2005 and 2006 Industrial Chemistry option questions are given in
the complete Answers to HSC exam papers which you can access from the CCHSC
home page (Click on it to go there). Answers
to exercises
260213 |