|
Further exercises
For pages 204–6
| A1. |
Sulfuryl
chloride, SO2Cl2 decomposes to form sulfur dioxide
and chlorine gas. A sample of sulfuryl chloride was placed in a container
under such conditions that at equilibrium 33% of the starting compound had
decomposed. |
|
(a) |
Draw
a graph showing the concentrations of SO2Cl2 and SO2
as functions of time until well after equilibrium had been reached. |
|
(b) |
At
time x, well after equilibrium had been established, the volume of
the reaction vessel was suddenly increased from 100 mL to 150 mL. Show on
your graph how concentrations would change at time x. Be
quantitative about this. Show how concentrations would change with time
after x as the system moved to re-establish equilibrium. Explain
why your curves have the shape and final equilibrium values that you have
given them. |
|
(c) |
Re-draw
the graph from part (a). Show as a dashed line the concentration of
chlorine as a function of time; you may have to displace this curve
slightly up or down to make it show up. Now suppose that at time x
additional chlorine had been added to the reaction vessel, sufficient to
increase its concentration by 50%. Show how the concentrations of the
three species would change at and after time x as the system
re-established equilibrium. Explain the shapes and final concentrations of
your curves. |
There are no B
exercises
For pages 223–4
(contains
some exercises involving back titration)
| C1. |
The
sulfate content of a fertiliser was measured by dissolving 1.63 g of
the fertiliser in 300 mL water then slowly adding a solution of barium
chloride until no further precipitation occurred. After filtration and
drying the mass of precipitate was 1.81 g. Calculate the percentage of
sulfate in the fertiliser. On the basis that the only source of sulfate in
this fertiliser was sulfate of ammonia (ammonium sulfate), calculate the
percentage nitrogen in the fertiliser.
|
| C2. |
A
certain brand of 'complete' fertiliser for home gardens claims to have
3.5% phosphorus 'present as water soluble phosphate'. To check on this
claim a group of students performed the following analysis. They dissolved
7.436 g of the fertiliser in water and filtered off the insoluble matter.
Magnesium chloride in an ammonia–ammonium chloride buffer was slowly
added to form a precipitate of Mg(NH4)PO4.6H2O.The
mass of precipitate formed, after filtering, washing and drying to
constant mass, was 2.060 g. Calculate the percentage P in the original
fertiliser. Is the claim on the packet correct?
|
| C3. |
0.462
g of a finely ground lawn fertiliser was mixed with 50.0 mL 0.196 mol/L
sodium hydroxide solution and gently boiled to expel the ammonia. After
cooling, the reaction mixture required 15.6 mL 0.134 mol/L sulfuric acid
solution for neutralisation. Calculate the mass of ammonium ion in the
sample, hence the mass of nitrogen in the sample and so the percentage
nitrogen in the fertiliser. For the final calculation assume that ammonium
ion is the only source of nitrogen in this fertiliser (generally not a
good assumption because many fertilisers also contain urea, NH2CONH2,
as a source of nitrogen).
|
| C4. |
Aspirin
is a weak monoprotic acid of molecular formula HC9H7O4.
To determine the amount of aspirin in a particular brand of headache
tablet an analyst ground up a tablet and dissolved it in 25.0 mL0.106
mol/L sodium hydroxide solution. After complete reaction the hydroxide was
titrated with 0.0958 mol/L hydrochloric acid solution. 10.33 mL was
required. Calculate the mass of aspirin present in the tablet. Why was
this procedure followed instead of just adding the ground-up tablet to
water and titrating it with sodium hydroxide solution?
|
| C5. |
The
concentration of citric acid in orange juice was determined by direct
titration. 50 mL (by pipette) of the orange juice was titrated with 0.213
mol/L sodium hydroxide solution using phenolphthalein as indicator.
Phenolphthalein normally changes sharply from colourless to red as pH
changes from 8 to 10. However the presence of the yellow colour of the
orange juice partly obscures this change. The equivalence point was taken
as the volume when a faint but distinct trace of red colour could be
detected. In successive titrations the end point occurred at 28.5 mL, 26.5
mL. 27.0 mL, 26.0 mL and 26.8 mL. Citric acid is a triprotic acid of
formula, C6H8O7.
|
|
(a)
|
Calculate
the molarity of citric acid in this orange juice and hence its percentage.
Include an estimate of the error in your values.
|
|
(b)
|
Suggest
a way (or ways) of overcoming the problems with this analysis.
|
|
(c)
|
Orange
juice also contains vitamin C which is a weak monoprotic acid: its formula
is C6H8O6. A typical concentration of
vitamin C (ascorbic acid) in orange juice is 45 mg/100 mL The titration
with sodium hydroxide determines total acid which in (a) we have
considered to be all citric acid. What extra percentage error has been
introduced by ignoring the vitamin C? Is this a serious problem for this
analysis? Explain.
|
For pages 230–2
| D1. |
Atomic
absorption spectroscopy was used to measure the concentration of iron in
several natural water samples. The samples were filtered then sprayed into
the flame of the instrument at a carefully regulated rate; absorbance by
the iron atoms produced in the flame was measured using the appropriate
lamp for iron. Results are tabulated below.
| Sample |
L |
M |
P |
Q |
| Absorbance |
0.74 |
0.05 |
1.05 |
0.28 |
To
convert absorbances into concentrations a calibration curve was
constructed as follows. 3.62 g hydrated iron(II) ammonium sulfate, a very pure
compound of iron, Fe(NH4)2(SO4)2.6H2O,
was dissolved in dilute acid solution and the volume made
up to 0.500 L. Volumes of this solution were accurately diluted to 1.00 L.
These diluted solutions were then analysed in the instrument in exactly
the same way as was used for the samples for analysis. results are
recorded below.
| Volume (in mL) of concentrated
solution diluted to 1.000 L |
1.00 |
2.00 |
5.00 |
10.00 |
| Absorbance |
0.07 |
0.13 |
0.34 |
0.69 |
Calculate the
concentration (in ppm) of iron in each of the standard solutions and draw
a graph of absorbance versus concentration. Use this to estimate the iron
concentration in each of the unknown samples. Are there any samples for
which it is difficult to estimate the iron concentration? Explain why.
Suggest a way of overcoming the difficulty.
Does this analysis measure iron(II) or iron(III) or both? Explain.
|
For page 244
| E1. |
Boron trifluoride reacts with
fluoride ion to form the boron tetrafluoride ion, BF4–.
Draw electron-dot diagrams for these three species. Explain why (or how) a
coordinate covalent bond needs to be used in the BF4–
structure. Can you identify which bond in the BF4–
structure is the coordinate covalent bond? Explain.
Suggest a reason why is the BF4– ion less reactive
than the BF3 molecule.
|
| E2. |
Anhydrous aluminium chloride
readily forms the AlCl4– ion.
Explain how the formation of this ion involves a coordinate covalent bond. |
For pages 252–3
| F1. |
Explain how the reactions 7.1 to
7.3 on page 248 cause the higher temperature that is observed in the top
half of the stratosphere (Figure 7.1 page 236) and why there is less
temperature increase in the lower half.
|
| F2. |
Name the following compounds |
|
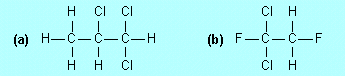
|
| F3. |
Draw structures for |
|
(a) |
1,2,3-trifluoropropane |
|
(b) |
1,1,3-tribromo-3,4-dichlorobutane
|
| F4. |
Draw the structure of and name
an isomer of each of the compounds in Exercises F1 and F2.
|
| F5. |
Which of the following compounds
if released to the lower atmosphere would cause most damage to
stratospheric ozone and which the least? Explain why. |
|
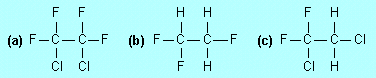 |
For page 256
| G1. |
Another pair of
reactions that occurs in the stratosphere is
HO + O3 ®
HO2 + O2
HO2 + O ®
HO + O2 |
|
(a) |
Is this a chain reaction? If so
which species is(are) the chain carrier(s)? |
|
(b) |
Draw electron dot structures for
HO and HO2. Would you consider these species to be molecules or
radicals? Why? Would you expect these two species to react with each
other? Why? What would you expect them to form? |
For page 270
| H1. |
Three farmers tested their bore water for total dissolved
solids (TDS) by measuring its conductivity. The samples had the values (a)
285 (b) 2075 and (c) 1105 mS m–1. Use Equation 8.1 on
page 268 to calculate the concentration of TDS in these samples. Comment on the
suitability of each sample for human consumption, stock watering and crop
growing.
|
| H2. |
Water in a particular stream had
a pH of 3.8, a TDS of 1650 ppm and a turbidity of 80 NTU. Is this stream
contaminated? If so what is the most likely source of the contamination?
Explain.
|
| H3. |
Some measurements on several water
samples are:
| Sample |
A |
B |
C |
D |
| pH |
7.3 |
4.9 |
6.6 |
6.4 |
| TDS (ppm) |
460 |
650 |
340 |
140 |
| Turbidity (NTU) |
10 |
70 |
45 |
5 |
Which of these samples would you
consider to be from
|
|
(a) |
a clean mountain stream |
|
(b) |
a stream after it had flowed
through an area that had recently been cleared of forest |
|
(c) |
stream polluted with
run-off from a mine site |
|
(d) |
an underground bore |
For pages 274–5 and 279
| J1. |
In the planning stages for a new
sewage works for a town on a small river, it was determined that a
dilution factor of 1 : 50 was possible (meaning that each litre of treated
sewage could be mixed with 50 L of river water). The BOD of the raw sewage
was expected to be about 200 ppm. To what level would the BOD need to be
reduced by the treatment works for the effluent to be able to decrease the
dissolved oxygen concentration by no more than 2 ppm? Explain.
|
| J2. |
To measure the hardness of a
town water supply a chemist performed the following analysis. Some
ammonia–ammonium chloride buffer was added to 100.0 mL of the water in a
conical flask (to produce a pH of about 11 which is required for the
titration to work). Two drops of Eriochrome Black T indicator were added
and the solution titrated with 0.0108 mol/L EDTA. The indicator changes
from purple to blue at the end point – as the last of the free Mg2+
ions are bound up as a complex with EDTA (see page 266). 3.5 mL was required. Calculate the total concentration (in moles per litre)
of magnesium plus calcium ions in the original water sample.
Hardness is commonly reported as the number of milligrams of calcium
carbonate per litre that is equivalent to the total number of moles of
calcium plus magnesium ions in the solution. Calculate the hardness of
this solution in these terms. Would you consider this water as hard or
soft? Why? |
For pages 284–5
| K1. |
Describe
the test you would perform or name the chemical species you would
test for to decide whether or not a sample of water
was contaminated with the following; state what you would observe if the
test were positive and if appropriate write an equation for the reaction involved. |
|
(a) |
heavy metals |
|
(b) |
excess salinity |
|
(c) |
fertiliser run-off |
|
(d) |
raw sewage |
|
(e) |
acid drainage from a coal mine |
|
(f) |
excess hardness |
|
(g) |
run-off from land that had
recently been cleared for farming |
| K2. |
To
determine the nitrate concentration in water samples a pair of students
followed a standard procedure which converted the nitrate in 25.0 mL
portions into a coloured solution. The absorbance of this solution at a
wavelength of 530 nm was measured with a colorimeter. Results from three
samples are shown below.
| Sample |
P |
Q |
R |
| Absorbance |
0.60 |
0.095 |
0.36 |
To calibrate the colorimeter (that is, to make it possible to convert
absorbances to concentrations) the students prepared standard solutions of
known nitrate concentration, put them through the same analysis procedure
then measured absorbances, following exactly the procedure used with the
unknown samples. To do this they dissolved 0.204 g potassium nitrate in
distilled water and made the volume to 1.000 L. They then diluted
different volumes of this solution to 1.000 L and then performed analyses
on 25.0 mL portions of these diluted solutions. The results are shown
below
| Volume (in
mL) of concentrated nitrate solution diluted to 1.000 L |
5.0 |
10.0 |
15.0 |
20.0 |
|
Absorbance
|
0.183 |
0.355 |
0.540 |
0.715 |
|
|
(a)
|
Calculate
the concentration in ppm (mg/L) of nitrate in each of the solutions used
in the calibration experiment. Draw a graph of absorbance versus nitrate
concentration. Draw a smooth curve or straight line (whichever seems more
appropriate) through the points, after deciding whether or not the origin
of the graph should be on the curve or line.
|
|
(b) |
Use
the line or curve drawn in (a) to determine the nitrate concentration in
each of the unknown samples. |
|
(c) |
Which,
if any, of these samples would you consider to be environmentally clean
with respect to nitrate? Which, if any, would you consider to be
significantly contaminated with nitrate. Suggest possible sources of this
contamination.
|
| K3. |
(a) |
The phosphate content of several
water samples from streams was measured as follows. Some ammonium
molybdate solution was added to 25 mL samples of the water to be tested.
This produced a pale yellow colour. Solid ascorbic acid and a suitable
catalyst were then added: this produced an intense blue colour. The
absorbance of this solution at 650 nm was then measured. Results are shown
below
| Sample |
W |
X |
Y |
Z |
| Absorbance |
0.81 |
0.08 |
1.27 |
0.18 |
To convert absorbance to concentration several
solutions of known phosphate concentration were put through the same
procedure. These measurements showed that under the conditions used
Phosphate ion concentration (in ppm) = 0.284 x absorbance
Calculate the phosphate ion concentration in each of the samples
above. |
|
(b) |
Discuss the possibility of algal
blooms occurring in these streams. |
Answers to Further exercises
| A1. |
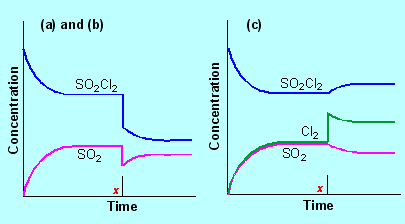
|
|
(b) |
The
equilibrium reaction is SO2Cl2  SO2 + Cl2
SO2 + Cl2
Increasing the volume from 100 to 150 mL decreases the concentration
of each substance by 33%. This causes a decrease in pressure in the
reaction vessel. By Le Chatelier's principle the reaction moves in
the direction which increases pressure; that is from left to right
(1 mole ®
2 moles) |
|
(c) |
When the
concentration of chlorine is increased, the reaction moves in the
direction which decreases it (Le Chatelier's principle), that is
from right to left, so more SO2Cl2 is formed.
|
| C1. |
45.7% sulfate,
13.3% nitrogen |
| C2. |
3.50%; yes |
| C3. |
0.101
g NH4+ ion; 0.0787 g N;17.0% N |
| C4. |
0.299
g; aspirin is insoluble in water so it reacts with NaOH only slowly.
In a direct titration we could easily overshoot the endpoint. A back
titration allows plenty of time for the aspirin to react with the
NaOH and then the excess is titrated with HCl which is a very fast
reaction. |
| C5. |
(a) |
Ignore the
first titration (it probably overshot the end point) and average the
other four;
( 0.038 ±
0.001)
mol/L; (0.73 ±
0.01)%(w/v) (error in the titre is about 2% so this is the
error in the answers) |
| (b) |
Perform the
titration using a pH meter to detect the end point (volume when pH
changes dramatically) |
| (c) |
Of the 5.66 x
10–3 mol NaOH used in the titration only 0.13 x 10–3
mol would have reacted with Vitamin C so the extra error is 2%. This
is comparable with the other errors in the analysis so it is not a
serious problem. |
| D1. |
In the
standard solutions concentrations are 1.032, 2.064, 5.161, 10.32 ppm.
L, 11.1 ppm; M, 0.8 ppm; P, 15.8 ppm; Q, 4.2 ppm; all ± 0.1 ppm
P may have been difficult to estimate because it is well outside the
calibration range; could dilute the sample 1: 2 and remeasure. M is
not particularly accurate because of its low absorbance.
The analysis measures total iron, because the flame converts all
species to atoms before analysis.
|
| E1. |

To make BF4– a
lone pair from the F– ion forms a bond with the boron.
This is a coordinate covalent bond. Boron does not have any valence
electrons left to share with the fourth fluoride ion which in turn
has no room in its valence shell for extra electrons.
In BF4– all four bonds are identical. We
cannot identify the one that was formed by this coordinate covalent
bond. The coordinate covalent bond is just a way of envisaging how
the bond forms: the final bond is no different from any other
covalent bond.
BF4– has a complete octet of valence
electrons whereas BF3 has only six valence electrons:
this makes BF3 quite reactive. |
| E2. |
Anhydrous
aluminium chloride is covalent. The formation of AlCl4–
can be envisaged in the same way as BF4–,
involving a coordinate covalent bond from a chloride ion to an AlCl3
molecule.
|
| F1. |
The reactions
convert short-wavelength ultraviolet radiation into heat which warms
up the atmospheric gases there; at lower levels there is much less
short-wavelength uv left (it was absorbed higher up) so there is
less absorption and less conversion to heat and so temperature does
not rise as much. |
| F2. |
(a)
1,1,2-trichloropropane (b) 1,1-dichloro-1,2-difluoroethane |
| F3. |
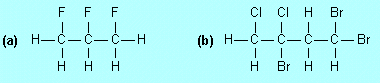 |
| F4. |
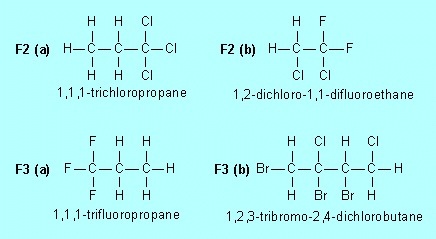
Many others
are possible. |
| F5. |
Most damage:
(a); no C–H bonds so it survives long enough in the atmosphere to
diffuse into the stratosphere, and it contains C–Cl bonds which
can be broken to release Cl atoms which destroy ozone.
Least damage: (b); no C–Cl bonds so if forms no Cl atoms in the
stratosphere, but with C–H bonds it is destroyed in the lower
atmosphere relatively quickly.
(c) is intermediate: much of it is destroyed before it gets into the
stratosphere (because of its C–H bonds) but what does get there
causes damage.
|
| G1. |
(a) |
yes; HO and
HO2 |
|
(b) |
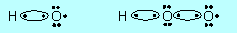
Radicals, because in each an O atom has
a lone electron, that is only 7 electrons in its valence shell.
Yes, because they can form two stable molecules H2O and O2
by having an H atom move from HO2 to HO:
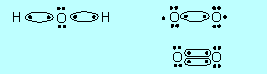 |
| H1. |
(a)
185 ppm (b) 1350 ppm (c) 720 ppm
(a) is suitable for all purposes; (b) not fit for human consumption,
acceptable for stock watering, would cause problems over time if
used for crop irrigation; (c) not suitable for human use (except for
short-term emergencies), suitable for stock, could be used for
crops, but would cause some damage, especially with prolonged use. |
| H2. |
yes; leaching
from a mine site or from disturbance of so-called acid-sulfate soils
by land clearing, farming, excavation or road making.
The measurements show that the stream has been contaminated by acid
(low pH), suspended matter (high turbidity) and dissolved salts
(high TDS). |
| H3. |
(a) D (low
pH, low turbidity and moderate TDS); (b) C (high TDS and suspended
matter but pH not affected); (c) B (low pH mainly, but high TDS and
suspended matter also); (d) A (moderately high TDS low turbiduty and
pH close to neutral)
|
| J1. |
100 ppm |
| J2. |
3.8 X 10–4
mol/L; 38 ppm; soft, because at this value the concentrations of
magnesium and calcium ions are too low to precipitate out soap as a
scum.
|
| K1. |
(a) |
add sodium
sulfide; if a precipitate forms, heavy metals are indicated. If no
precipitate forms, add some alkali and check again: some metal
sulfides precipitate only in alkaline solution (page 270) |
|
(b) |
measure total
dissolved solids (with a conductivity meter)
OR if it is sodium chloride that you are looking for add silver
nitrate solution; a white precipitate forms.
Ag+(aq) + Cl–(aq) ®
AgCl(s) |
|
(c) |
Test for
phosphate or nitrate |
|
(d) |
Test for
coliform bacteria (page 265)
OR test for BOD |
|
(e) |
measure pH
(lower than 6 indicates some contamination by acid |
|
(f) |
Titrate with
EDTA (page 266-7) |
|
(g) |
Measure
turbidity: a value above 20 NTU indicates serious contamination by
suspended matter. Could also measure TDS.
|
| K2. |
(a) |
).625, 1.25,
1.88, 2.50 ppm; points lie on a straight line through the origin |
|
(b) |
P, 2.1; Q,
0.32; R, 1.25 ppm (all ± 0.05 ppm) |
|
(c) |
Clean: Q; P
is definitely contaminated with nitrate; R is marginal – 1 ppm is
about as high as nitrate gets in uncontaminated water, but R is only
just above this. The most likely source of the nitrate in P is
fertiliser run-off from farm land: it could also come from treated
sewage or from treated effluent from a chicken farm or animal
feed-lot. |
| K3. |
(a) |
W, 0.23; X,
0.023; Y, 0.36; Z, 0.051 |
|
(b) |
With
phosphate concentrations above 0.1 ppm, streams W and Y are quite
susceptible to algal blooms. If the water in stream Z is stagnant
(hardly flowing) it could also be susceptible. There is little risk
that X would develop an algal bloom.
05022014
|
|