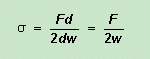|
The syllabus and Conquering Chemistry For Module 3 Conquering Chemistry Preliminary Course
4th edition follows the syllabus very closely both in content and in sequence of
presentation as can be seen from the Module
3 and the New South Wales HSC syllabus tables on pages 235–8. Three minor
deviations are (i) the inclusion of the structure of methane
and dispersion forces (for comparison
with structures of water, ammonia and hydrogen sulfide in order to illustrate
the effects of different intermolecular forces upon properties), (ii) discussion
of water of crystallisation and hydrates in Chapter 7 and (iii) mention of some
other reversible reactions (in addition to the precipitation and dissolution
required by the syllabus) in Chapter 8.
Conquering Chemistry uses the term
neutral species equation for equations such as Equation 8.4 on page 206
whereas the syllabus document (Section 9.5.4) uses the term full formulae
equation for such equations. Neither term is
completely satisfactory, so teachers and students should feel free to use
whichever term they prefer. The important thing is to
recognise that there are three types of equations for ionic reactions, complete
ionic, net ionic and the one that writes the ions as parts of neutral compounds. 2. Introduction to equilibrium If equilibrium is introduced before concentration (as is done in the syllabus), care is needed not to use concentration in explaining equilibrium! The only points about equilibrium that need to be introduced here are
But remember rate of reaction has not been formally introduced at this stage! Le Chatelier's principle need not be introduced until Module 1
of the HSC course. 3. Different measures of concentration The various measures of concentration listed on pages 212–3 seem
to be required by the syllabus, though they were not in the old syllabus. The reason for deferring
discussion of ppm for gases till later is that it needs the molar volume of
gases (to interconnect molecules per million molecules and volumes per million
volumes) which is not introduced until Module 2 of the HSC course. 4. Exothermic reactions get hotter?? Some students have difficulty with the idea that when an exothermic reaction is performed in a test tube the test tube gets hot: they see this as the test tube and its contents gaining heat not losing it and so shouldn't the reaction be endothermic?. Hence it is necessary to be very careful in describing exo- and endothermic reactions. In exothermic reactions there is a loss of energy (chemical energy) by the reactants as they form products and this energy is given out as heat and this heat warms up the test tube and its contents. The process is chemical energy being converted to heat energy. Similarly with endothermic reactions: as they occur the reactants need to take in energy (to convert to chemical energy) to store in the products. Generally the only place that the reaction can get the required energy is from the heat in the test tube and its contents, so the tube and contents get cold: heat energy is being taken out of the substances and converted to chemical energy stored in the products of the reaction. It is perhaps unfortunate that
enthalpy (next module, page 276) is often described as 'heat content' (but not
in CCPC 4th edition) rather than as the total energy contained in a substance.
Despite how enthalpy is defined, the change in enthalpy is correctly
defined as heat absorbed at constant pressure. The Hreactants
and Hproducts in Figure 10.1, page 276, are really chemical
energies rather than heat contents. When there is an increase in chemical
energy, DH
positive, the energy is obtained by extracting heat from the substances and so
the reaction mixture gets cold. And when there is a decrease in chemical energy, DH
negative, the released chemical energy appears as heat and so the reaction
mixture gets hot. The syllabus introduces this equation in Section 8.4.5 but does not state what DH stands for. Generally in chemistry texts DH means change in enthalpy, but enthalpy is not introduced until Module 4. The quantity m C DT is a measure of the heat absorbed or the heat released when a body or substance undergoes a change in temperature, DT. If we define DT as the increase in temperature as the sample goes from its initial state to its final state, then m C DT will be the heat absorbed by the sample. Chemistry books generally use q for the heat absorbed. So the correct equation isq = m C DT ...(5.1) This is Equation 8.11 on page 223. If the temperature of our sample increases (DT positive), then q is positive; that is heat is absorbed by our sample. If the temperature of our sample decreases (DT negative) then q is negative. If a negative amount of heat is absorbed, that must mean that heat is released. How does DH get into this equation? As we shall see in Chapter 10, DH is the symbol used for the change in enthalpy for a chemical reaction (or other process such as the dissolution of a substance in a solvent). DH is defined as the heat absorbed when the change occurs at constant pressure. As we shall also see in Chapter 10 we often measure DH by measuring the change in temperature in a quantity of water or solution in which the reaction (or dissolution) being examined has occurred. For example we determine the heat of solution of a solid in water by measuring the change in temperature in the water when a known mass of solid is dissolved in the water. Suppose the temperature increases as the solid dissolves. We calculate the amount of heat absorbed by the water from the above equation. This heat absorbed by the water must be the amount of heat released by the dissolution. So the amount of heat absorbed by the dissolution (or reaction) must be minus this quantity. That is: Amount of heat absorbed by the dissolution or reaction = – m C DT If the process occurred at constant pressure (that is in a container open to the atmosphere), then the heat absorbed is DH; that is DH = – m C DT ...(5.2) DH is the enthalpy change for the process (reaction or dissolution) occurring, while m C DT is the heat absorbed by the substance receiving the heat from the process. Generally DH is quoted per mole of specified substance. What we have so far is the enthalpy change for the amount of substance dissolved or reacted in our experiment so we need to convert it to an amount per mole. If the experiment was not performed at constant temperature, for example if it was performed in a closed container and involved production or loss of a gas, then the heat released by the reaction (that is the heat absorbed by the water or solution) would not be DH. Hence Equation 5.2 applies only under very restricted conditions. On the other hand Equation 5.1 here (or 8.11 in the book) is much more widely applicable; it applies whenever a sample undergoes a temperature change. Example 13 illustrates. It may be clearer if after 'From Equation 8.11' it had read 'heat released by dissolution of NaOH = heat absorbed by the solution = 211.2 x 4.2 x (31.4 – 19.2)'
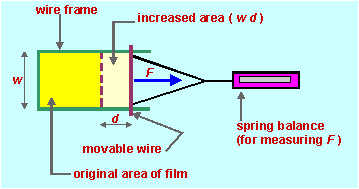
As in previous modules most of the material in this section is not required by the HSC syllabus. It is presented here either because it provides better understanding of syllabus material or because of its inherent interest to HSC students. 1. Surface tension and viscosity The definitions in Conquering Chemistry page 184 are adequate for HSC purposes, but to understand how these quantities get the units they have, we need more precise definitions. Surface tension is the energy (work) required to increase the area of a surface by unit area. It can be measured by making a film of liquid (e.g. a soap bubble) on a frame with a movable side as in the following diagram.
A sensitive spring balance is used to increase the area of the film (pull on
the movable wire) by a measured amount. The force required is measured by
the spring balance, and the distance moved by the wire and the width of the film
are
measured. The work done is F x d and the increase in area of the film is 2
x d x w (2 because the film
in this case has two sides). Surface tension
s
is work done per unit area so In a liquid flowing across a stationary surface or through a tube, viscosity is the force per unit area needed to maintain a unit velocity gradient perpendicular to the direction of flow. In the diagram below we have a liquid (e.g. treacle) sandwiched between two plates. One plate is stationary while a force Fx is applied to move the other plate.
The movement of one plate relative to the other sets up a velocity gradient: liquid near the stationary plate is not moving while liquid near the moving plate has the same velocity as the moving plate. The velocity gradient is dv/dz. If the area of the moving plate in contact with the liquid is A, then viscosity h is given by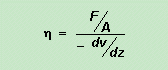 (minus because dv/dz is negative with the origin for x and z defined as in the diagram). F/A has the units N m–2 and dv/dz has the units m s–1 m–1. This gives viscosity h the units N m–2 (m s–1 m–1)–1 = N m–2 s = Pa s where Pa is a pascal (unit of pressure, and equal to N m–2 ). Again because of the magnitude of common viscosities, they are often given in millipascals seconds, mPa s. Older books may use the Poise as the unit of viscosity. 1 Poise = 0.1 Pa s. Despite what was said about saturated solutions on page 208, it is sometimes possible to obtain solutions which contain more solute per unit volume of solvent than is ‘allowed’ by the solubility! If a saturated aqueous solution of sodium thiosulfate (photographer’s hypo or fixer) is prepared at 80 or 90°C, it will contain about 70 g/100 g water. If it is slowly cooled to room temperature, it stays as a clear solution. Although at 25°C the solubility is only about 40 g/100 g, the excess thiosulfate does not crystallise out. Such a solution is said to be supersaturated. A supersaturated solution is one that contains a greater concentration of solute than is expected from the solubility. Such supersaturated solutions are
not particularly stable. The excess solute can be made to crystallise out
sometimes by vigorously shaking the solution, or scratching the walls of the
container with a sharp rod, or sometimes just by letting some dust from the air
settle on its surface. The best way to crystallise out the excess solute is to
add a few small crystals of the solute to the solution. These act as ‘seed’
crystals which quickly cause the excess solute to crystallise out. Supersaturated
solutions cannot exist in contact with crystals of the pure solute. This
then is the difference between a saturated solution and a supersaturated
one: Honey is an everyday example of a
supersaturated solution. It is a supersaturated solution of glucose and fructose
(common sugars). If it is seeded with crystals of glucose or fructose, as often
inadvertently happens if some honey around the mouth of the jar dries out to
form crystals, the excess solid crystallises out and forms candied honey. It can
be clarified again by heating in hot water. At the high temperature, the amounts
of glucose and fructose present can all dissolve without supersaturating the hot
solution. Once all the solids have dissolved the honey can be slowly cooled.
Provided care is taken to remove all traces of solids (seed crystals) from
around the mouth of the jar, the honey will remain clear, that is
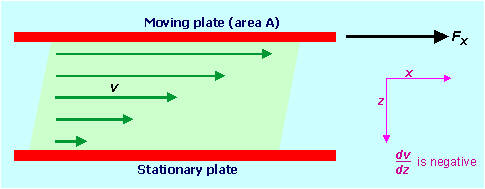
supersaturated. 3. Conductivity of solutions; electrolytes and non-electrolytes The syllabus does not specifically mention conductivity of solutions or the terms, electrolyte, strong and weak electrolyte, non-electrolyte, and so these are not discussed in Conquering Chemistry Preliminary Course, although passing reference is made to conductivity of solutions of ionic and covalent substances on pages 57–9. Because these concepts are used in the HSC course, perhaps they should be discussed in this module. If the apparatus shown in the figure below is used to measure the relative conductivity of some common solutions, the results shown in the table are obtained. Relative conductivity in this experiment would be the current measured on the ammeter for a constant voltage from the battery and constant area, separation and depth of immersion of the electrodes in the test solution; the test solutions should have approximately the same concentration. Some solutions do not conduct electricity at all (left-hand column), some have relatively low conductivities (middle column), while others have relatively high conductivities (right-hand column).
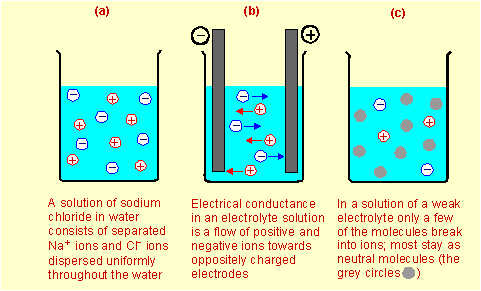
On the basis of these results, the technical term electrolyte is introduced. An electrolyte is a substance which in solution conducts electricity. Substances which in solution do not conduct electricity are called non-electrolytes. Substances in the middle and right-hand columns of the table are electrolytes. The substances in the left-hand column are non-electrolytes. The good conductors (right-hand column) are called strong electrolytes, while those which conduct, but not very well (middle column), are called weak electrolytes. The reason some solutions conduct electricity is that the solutes, instead of being made up of neutral molecules like sucrose, urea or iodine, are made up of positive and negative particles called ions. As was explained in Section 2.15 on pages 49–51, solid sodium chloride, for which the formula is NaCl, consists of positive Na+ ions and negative Cl– ions packed into an orderly array in the salt crystal. This was shown in Figure 2.11 (page 50). When crystals of sodium chloride dissolve in water, they break up into individual ions which move freely and independently through the solution, as shown in Figure 7.10 (page 195) and in Figure (a) below. When electrodes (electrical conductors) are placed in the solution, the positive ions are attracted towards the negative electrode and the negative ions are attracted towards the positive electrode as shown in Figure (b). This flow of ions through the solution constitutes an electric current and therefore the solution is a good conductor.
Strong electrolytes are made up of positive and negative ions which become completely dispersed in aqueous solution. Some substances such as acetic acid (in vinegar), CH3CO2H, exist in the pure liquid state as neutral molecules, but when they are dissolved in water, some of the molecules react with water to form positive and negative ions (though the vast majority remain as neutral molecules). Of every 100 molecules of acetic acid dissolved in water, typically 99 remain as CH3CO2H molecules while the other one forms a positive H3O+ ion (sometimes written just as H+) and a negative CH3CO2– ion as shown in Figure (c) above. These ions cause the solution to have a small but significant electrical conductivity. Substances in which only a small fraction of the molecules break up into positive and negative ions when they are dissolved in water are called weak electrolytes. This is in contrast to strong electrolytes which exist in solution totally as ions. Exercises based on these ideas are under the Page
212 sub-heading in Further exercises
below (F5 and F7).
|