|
The major way in which Conquering Chemistry deviates from syllabus order in this module is in treating stoichiometry before the Periodic Table. The reason for this is that a historical development of the Periodic Table as required by the syllabus needs atomic weights. It would be very artificial to introduce atomic weights simply as a prelude to discussing the history of the Periodic Table. The logical place to introduce atomic weights is in a discussion of quantitative aspects of equations and formulae (stoichiometry). Therefore it would appear preferable to treat moles and stoichiometry before the Periodic Table (that is treat Section 8.3.4 on p.31 of the 2002 syllabus document before Section 8.3.3 on p.30 ). The way that CCPC relates to the HSC syllabus is shown in the Module
1 and the New South Wales HSC syllabus tables on pages 175–8. 1. Energy considerations in extracting metals It is sometimes argued that because energy must be supplied to break a metallic compound into its constituent elements, energy is required to extract a metal from its ore. This is not a valid argument, because often the chemical reaction used to extract the metal from its ore is not a simple decomposition reaction (for example Cu from Cu2S + O2 ® 2Cu + SO2 or Fe from Fe2O3 + 3CO ® 2Fe + 3CO2) and so it may require the input of energy or may release energy. However it is true that energy is required to extract metals from their ores because of the reasons set out at the bottom of page 113. For some metals, for example aluminium and magnesium, a large input of energy is required to make the extraction reaction occur (electrolysis in both of these examples) and this adds significantly to the energy costs of such metals (see Table 4.7 on page 114).Several terms are used for roughly similar concepts:
(and analogous terms with molecular replacing atomic) Relative atomic mass is the 'correct' term for what has historically for over 150 years been called atomic weight – a relative mass, a dimensionless quantity. The atomic weight of sodium is 23 (not 23 u or m or grams)Atomic mass is the mass of an atom: it is a mass: 3.8 ´ 10–23 g for sodium or 23 u or 23 amu where u or amu is 1.66 ´ 10–24 g.Molar mass is the mass of one mole, 23 g/mol for sodium. It is the term that is really needed in stoichiometric calculations. For compounds similar terms exist; for example for carbon dioxide, 6.02 ´ 1023 particles per mole (or just mol–1) is the Avogadro constant - it's not a number, because it has units.While there is probably no need to be pedantic about this with students,
perhaps as teachers we should be precise in the words and terms we use. 3. Limiting reagent and mass-volume calculations? These are not required here. Mass-volume calculations are in Module 2 of the
HSC course. However it's a bit hard to know just what is required for 'process
information from secondary sources to investigate the relationship between the
volumes of gases involved in reactions involving a metal and relate this to an
understanding of a mole' (2002 syllabus document page 31). I have taken this to
mean the type of volume–volume calculation done on pages 151–2 and in the
exercises that follow. Two such reactions involving metals are used there
(Example 12 and Exercise 44(b)); others are Fe2O3 + CO
forming CO2 and CuO + H2 forming steam. Alloys are made by placing the required masses of the different metals in a suitable container and heating the mixture until it is completely molten: this forms a normal liquid solution. After allowing time for the mixture to become homogeneous right down to the atomic level, it is cooled. It freezes to a homogeneous solid mixture. Melting the mixture and allowing it to cool and solidify is the only way of obtaining a homogeneous mixture of two or more solids, that is to make an alloy. Fortunately students are no longer required to make an alloy. 2. Relating uses to properties (Section 4.3) Another example of relating uses to properties is the following. Copper Copper is easily extruded into tubes or pipes and is sufficiently soft for such pipes to be easily bent. These properties plus its high resistance to corrosion and the ease with which it can be soldered lead to copper being widely used for plumbing in houses. Copper forms several very important alloys, notably brass and bronze. Brass is significantly harder than copper, but still resists corrosion. This is why brass is used for making plumbing fittings such as taps, shower heads and tee junctions.
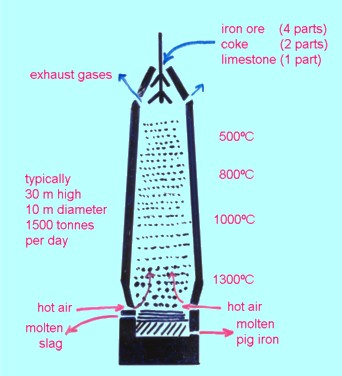
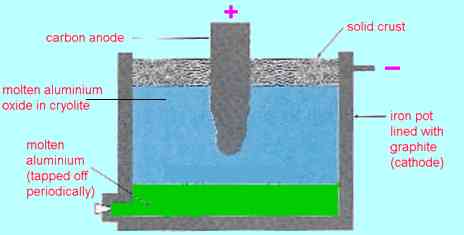
3. Extraction of metals from ores (Section 4.5 to 4.7) It seems a pity that only copper is required for the HSC, particularly as its chemistry is quite complex. If you want to look at the extraction of other metals which are perhaps more representative of general methods, here are iron and aluminium. Extraction of iron . A mixture of iron(III) oxide, coke (carbon) and limestone (impure calcium carbonate) is added to the top of a tower-like structure (about 30 m high) with hot air blasted in from the bottom. The carbon is converted to carbon monoxide which reduces the iron(III) oxide to iron: Fe2O3(s) + 3CO(g) ® Fe(l) + 3CO2(g) The limestone decomposes to calcium oxide which combines with silica (from impurities in the ore) to form slag which floats on top of the molten iron that collects at the bottom of the furnace. The product from the blast furnace is called pig iron or cast iron. It contains about 3 to 4% carbon. This is the end of Step 3 in Figure 4.1 (page 110). Steel is made by melting cast iron and bubbling oxygen through it to burn off most of the carbon. Alloy steels are made by adding measured amounts of other metals such as chromium, nickel, vanadium or tungsten after the carbon has been burnt off. This is Step 5 of Figure 4.1 (page 110). Extraction of aluminium Aluminium is too reactive a metal (Section 4.11 on page 119) for aluminium oxide to be reduced by carbon or carbon monoxide, so it has to be extracted by electrolysis. As we saw in Section 2.22 on page 57 ionic substances conduct electricity (and therefore undergo electrolysis) when they are molten. Unfortunately aluminium oxide does not melt until the temperature is above 2000oC which is too high for electrolysis to be practical. However a mixture of cryolite, K3AlF6, and aluminium oxide melts at about 1000oC. Electrolysis is possible at this temperature. The overall process can be written as 2Al2O3(l) ® 4Al(l) + 3O2(g) (electrolysis)with the oxygen eating away the graphite (carbon) positive electrode: C(s) + O2(g) ® CO2(g)This is Step 3 of the extraction process. Diagrammatically:
Virtually pure molten aluminium is removed from the electrolysis cell. While much aluminium is used pure, significant amounts are alloyed with small amounts of other metals for specialised uses. Iron and aluminium are unusual in that their ores are so rich in the metals
that no concentration of the ores (Step 2) is necessary. Copper ores have much
lower metal contents and so concentration is needed. 4. Oxidation and reduction and half reactions (Section 4.10) Not strictly in the Preliminary Course, but seems odd not to introduce the terms oxidation and reduction particularly when writing 'half equations to represent the electron transfer reactions occurring …' The terms oxidation and reduction were originally introduced in connection
with metals. Reduction was used to describe the extraction of a metal from its
ore (usually an oxide or sulfide): the ore was reduced to the metal.
Oxidation was originally used for the corrosion of metals because corrosion
generally meant reaction with oxygen. So gain of oxygen was called oxidation.
Nowadays these terms have more general use: they are applied to all elements,
not just metals, and they involve processes other than just loss or gain of
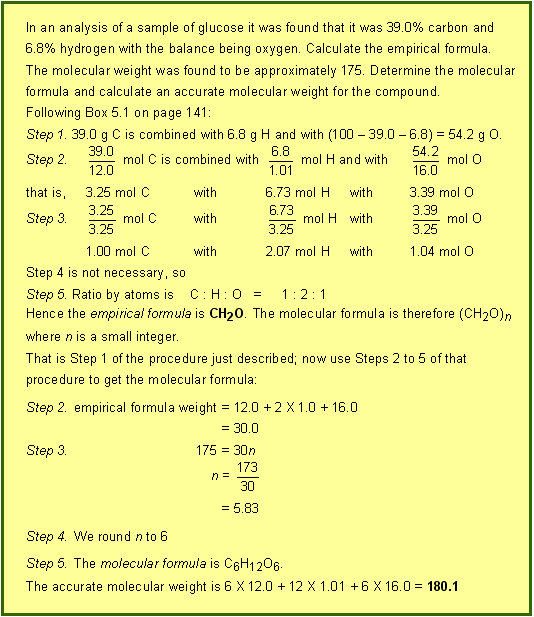
oxygen. 5. Calculating formulae from experimental data (Section 5.8) Calculating molecular formulae from empirical formulae appears not to be required for the HSC any more. However if you still want to do it, then continuing from the tbottom of page 141: A chemical analysis gives us the empirical formula for a compound. This is the only formula for ionic and covalent lattice compounds. However for molecular compounds we want the molecular formula. This requires some extra information such as an estimate of the molecular weight. Formulae for molecular compounds Such experimental measurements show that the molecular weight of ethene is 28 whereas a chemical analysis shows that its empirical formula is CH2. If the empirical formula of ethene is CH2, the molecular formula must be (CH2)n, where n is the integer we want to determine. If the molecular formula is (CH2)n, then the molecular weight is: n (12.0 + 2 ´
1.01) = 14n Therefore the molecular formula is C2H4. We sometimes use the term empirical formula weight for the sum of the atomic
weights in the empirical formula. The empirical formula weight of ethene is molecular weight = n ´ (empirical formula weight) where n is the small integer described above. In summary: To calculate a molecular formula from an approximate molecular weight 1. Calculate the empirical formula (Box 5.1 on page 141) 4. Round off n to the nearest integer (errors of up to 10% are quite normal) 5. Multiply each of the subscripts in the empirical formula by n to get the molecular formula An example will consolidate these ideas.
260213 |