|
Further
exercises
For pages 8–9
| A1. |
Some samples of air
are homogeneous while others are heterogeneous. Explain how this is
possible.
|
| A2. |
(a) |
Can a mixture be
homogeneous? If so give an example. Explain fully. |
|
(b) |
Can a pure
substance be heterogeneous? If so give an example. Explain fully.
|
| A3. |
When some silvery
aluminium turnings were mixed with powdered yellow sulfur and the mixture
heated, a white homogeneous solid formed. On cooling it remained as a
white solid. When heated again, it underwent no apparent change. When the
experiment was repeated several times using different masses of aluminium
turnings and sulfur, the white solid always contained 36% aluminium. Is
the white solid a mixture or a compound? Explain why.
|
For page s
s
15 and 18–19
Some of the exercises
in this set require techniques described in the Supplementary Material section
above.
| B1. |
Brandy (about
40% ethanol) is made by distilling wine (12 to 14% ethanol). In such a
distillation is the brandy the distillate or is it the liquid left in the
distillation flask? Explain. If you distilled some brandy, would the
distillate contain a higher or lower percentage of ethanol than the original
brandy? Explain.
|
| B2.2. |
A student
accidentally poured an aqueous solution of silver nitrate into a bottle of
kerosene. How would you recover the aqueous solution?
|
| B3. |
When air (after
removal of moisture and carbon dioxide and impurities) is cooled to –210oC,
a homogeneous liquid mixture (solution) of nitrogen and oxygen forms. If
this mixture is slowly warmed until it starts to boil, what would you expect
the vapour formed to be – pure oxygen, pure nitrogen, or a mixture? If a
mixture how would its composition compare with that of the liquid? How could
you obtain a sample of one pure substance from the original liquid and what
substance would it be? Again use Table1.8 on page 23 to answer this
question. Note that there should be minus signs in front of the boiling
points of oxygen, nitrogen and hydrogen, that is –183, –196 and –253oC.
|
| B4. |
In a school
laboratory a new supply of small lead pellets was accidentally put into a
jar containing iron filings. How would you separate the lead from the
iron? More than one way may be possible.
|
| B5. |
How would you
separate a mixture of (a)
sand and iodine (neither has a significant solubility in water) (b)
sodium chloride and ammonium chloride (both are soluble in water).
|
| B6. |
When a solution
of ammonia was added to a solution of copper sulfate, the resulting mixture
had an intense dark blue colour. When a solution of sodium hydroxide was
added to a solution of copper sulfate, the resulting mixture had a pale blue
milky appearance. Both mixtures remained unchanged for many minutes. Then
both mixtures were centrifuged. The deep blue mixture remained unaltered,
but the pale blue one separated into a clear solution and a pale blue solid
at the bottom of the centrifuge tube. Which, if either, of the original
mixtures was heterogeneous and which, if either, was a solution? Explain
why.
|
| B7. |
How would you
separate a mixture of carbon, iodine and ammonium chloride. Ammonium
chloride is readily soluble in water but insoluble in hexane; iodine is
readily soluble in hexane but only slightly soluble in water while carbon
is insoluble in both solvents.
|
For page 22
| C1. |
(a) |
At room temperature
(20oC), which of the substances in Table 1.7 on page 21 are (i)
solid (ii) liquid (iii) gas? |
|
(b) |
Which if any of
these substances would undergo a change of state if the temperature was (i)
lowered to –2oC (ii) raised to 100oC?
|
For page 23
| D1. |
To determine the
density of lead a pair of students took some lead shot (pellets),
determined their mass then poured them into a burette containing some
water. They noted the reading on the burette before and after adding the
lead pellets. Use their results below to determine the density of lead. |
|
Mass
of lead pellets taken |
= 142.6 g |
|
Initial
reading of the burette |
= 34.7 mL |
|
Burette
reading after adding the lead shot |
= 22.2 mL
|
| D2. |
Explain how you
would determine the density of a fine gold chain (necklace). How could you
use your answer to decide whether the chain was solid gold or just gold
plated copper. You may use data in Table 4.4 on page 104.
|
For page 25
| E1. |
Each tablet of the
Alka Seltzer shown in the photo on page 24 contains
324 mg aspirin, 1.9 g sodium bicarbonate and 1.05 g citric acid. Calculate
the per cent composition of this mixture.
|
| E2. |
To determine the
composition of bagged dry concrete mix (crushed rock, sand, cement),
a pair of students used a set of shop scales to weigh out a sample of the
mixture (3.23 kg). They then used a coarse sieve to separate out the
crushed rock (aggregate) then weighed the separated rock (1.85 kg). They
then used a fine sieve to separate the sand from the cement. Using
laboratory scales they found that the sand has a mass of 910 g and the
cement 420 g. Determine the percentage composition of the dry concrete
mix.
|
| E3. |
In Exercise E2
above why do you think the sum of the masses of the separated components
was less than the starting mass? Suggest another source of error in this
experiment. How would it affect the results? How could the experiment be
modified to give more accurate results?
|
| E4. |
Chalcocite is a
mineral of copper (not a common one in Australia). It is a compound
containing copper and sulfur. When carefully heated in air black
chalcocite decomposes to reddish brown copper (with the sulfur vaporising
as sulfur dioxide). A 2.36 g sample of chalcocite formed 1.89 g copper.
Calculate the percentage copper in chalcocite.
|
| E5. |
Brass is an alloy
(solid solution) of copper and zinc. To determine the composition of a
sample of brass filings a chemist mixed 1.72 g of the filings with warm
hydrochloric acid; this dissolved the zinc but left the copper unaffected.
After complete reaction (no further evolution of gas) the remaining solid
was filtered off and weighed: it had a mass of 0.92 g. Calculate the
percentage copper in that particular brass.
|
| E6. |
The experiment in
Exercise E5 was repeated on three other samples of brass. Use the results
below to show that brass is a mixture and not a compound. |
| Mass of sample used (g) |
1.38 |
2.04 |
1.87 |
| Mass of copper left (g) |
0.81 |
1.00 |
1.05 |
There
are no F
exercises
For page 39
| G1. |
Molecular
formulae for some everyday substances are given below. |
|
(a) |
How
many atoms of each type are present in a molecule of each of these
substances? |
|
(b) |
What is
the total number of atoms in each of these molecules? |
|
|
(i) |
boracic acid or
boric acid (disinfectant), B(OH)3 |
|
|
(ii) |
acetic (ethanoic
acid) (in vinegar), CH3COOH |
|
|
(iii) |
urea (common
nitrogenous fertiliser), CO(NH2)2 |
|
|
(iv) |
ascorbic acid
(Vitamin C), C6H4O2(OH)4
|
| G2. |
Write
molecular formulae for the compounds below. The number of each type of
atom present in the molecule is given: |
|
(a) |
Refrigerant 134a
(currently used in air conditioners); 2 carbon,
2 hydrogen and 4 fluorine atoms |
|
(b) |
Cysteine, on of the
essential amino acids; 3 carbon, 7 hydrogen,
2 oxygen 1 sulfur and 1 nitrogen atoms |
|
(c) |
peroxyacetyl
nitrate, a constituent of photochemical smog; 2 carbon,
3 hydrogen, 1 nitrogen and 4 oxygen atoms
|
For pages 42–3 and
45
| H1. |
What
are the atomic and mass numbers of the element in which the atoms contain |
|
(a) |
14 protons and 15
neutrons |
|
(b) |
42 neutrons and 33
electrons |
|
(c) |
22 neutrons and 18
protons |
|
(d) |
12 electrons and 12
neutrons |
|
Name,
and give the symbol for, each of these four elements.
|
| H2. |
Two atoms each have
12 protons in the nucleus: one has 12 neutrons while the other has 13. How
many electrons do each of these atoms contain? Do these two atoms belong
to the same or different elements? Explain.
|
| H3. |
Using Figure 2.9(b)
on page 53 as a guide, give the electron configuration of the following elements
(atomic number in brackets): Sr (38), Zr (40), Tc (43), Sb
(51), Xe (54).
|
| H4. |
Give the electron
configuration of O, S, Se and Te for which the atomic numbers are 8, 16,
34 and 52. What common feature is there about these four configurations?
|
For page 49
| J1. |
Write down the
electron configurations of elements having atomic numbers
9, 3, 12, 18, 15, 4, 20, 30, 13, 36, 6, 16. State which group of the
Periodic Table each belongs to.
|
| J2. |
Use the Periodic Table to
predict how many electrons there are in the outermost energy level (shell)
of the following atoms:
barium, bromine, gallium, arsenic, caesium, selenium
|
| J3. |
We often talk about the
electron configuration of monatomic ions. To obtain the electron
configuration of an ion, we start with the configuration of the atom and
add or subtract the necessary number of electrons to form the ion. We add
electrons to the next available positions in the energy levels and we
remove them from the highest energy level (last in, first out!). Hence
give the electron configuration of the following, taking atomic numbers
from the Periodic Table if necessary |
|
(a) |
potassium atom,
potassium ion |
|
(b) |
fluorine atom,
fluoride ion |
|
(c) |
aluminium atom,
aluminium ion |
|
(d) |
sulfur atom,
sulfide ion
|
| J4. |
(a) |
Write
down the electron configuration of the members of each of the following
sets of atoms and ions: |
|
|
(i) |
O2–, F–,
Ne, Na+, Mg2+ |
|
|
(ii) |
S2–, Cl–,
Ar, K+, Ca2+ |
|
(b) |
What do
all five species in each set have in common? Why is this so?
|
For pages 52 and 56
| K1. |
Draw diagrams
similar to those in Examples 1 and 2 on pages 50–1 to show the
formation of ionic bonds involving
(a) lithium and
bromine
(b) magnesium and sulfur
(c) sodium and oxygen
|
| K2. |
Using a Periodic
Table, deduce the electron configuration of, and the charge on, the ions
you would expect to be formed by: |
|
(a)
strontium
(b) iodine |
(c) rubidium
(d) selenium
|
| K3. |
Draw electron dot
diagrams and give the molecular formulae for covalent molecules formed
between
(a) chlorine and
iodine
(b) hydrogen and sulfur
(c) phosphorus and fluorine
|
| K4. |
Hydrogen and
nitrogen are able to form the negative hydride and nitride ions
respectively. Draw electron dot structures for these two ions, showing
clearly the charge on each. Sodium can form both a hydride and a nitride;
what formulae do you expect for these compounds?
|
| K5. |
Which
of the following compounds would you expect to be ionic? Explain why, and
draw electron dot diagrams of the ions present, and give the formulae of
the compounds: |
|
(a) magnesium
chloride
(b) sulfur dichloride
(c) barium oxide
(d) nitrogen triiodide
(e) sodium sulfide |
(f) boron
trifluoride
(g) calcium chloride
(h) potassium iodide
(i) oxygen fluoride
(j) iodine chloride
|
| K6. |
Which of the
compounds in Exercise K5 would you expect to be covalent? Draw electron-dot
diagrams for them and give their molecular formulae.
|
For pages
59 and 63
| L1. |
Arsenic tribromide
and magnesium bromide are white solids at room temperature. The solids
melt at 31oC and 711°C respectively. As liquids, magnesium
bromide conducts electricity while arsenic tribromide does not. Explain
the difference in melting points and conductivities in terms of the
bonding in the two substances.
|
| L2. |
Tungsten carbide (carborundum)
is an extremely hard substance (comparable to diamond) with a very high
melting point, 2870oC. it does not conduct electricity and is
insoluble in all common solvents. What do you conclude about its
structure?
|
| L3. |
Tin
forms two distinct compounds with chlorine, SnCl2 and SnCl4.
SnCl2 is a
solid at room temperature while SnCl4 is a liquid. The melting point of
SnCl2 is 247°C and the boiling point of SnCl4 is 113°C. Liquid
SnCl2
conducts electricity while liquid SnCl4 does not. |
|
(a) |
What do you conclude
about the bonding in SnCl2 and SnCl4? Give your
reasoning. |
|
(b) |
For any ionic
compound(s) state what ions you expect to be present. For any covalent
compound(s) draw an electron-dot diagram. Again give reasons for your
conclusions.
|
| L4. |
Use the
Periodic Table to answer the following: |
|
(a) |
Sodium chloride, oxide, fluoride
and sulfide have the formulae, NaCl, Na2O, NaF, Na2S.
What do you expect to be the formulae of:
(i) rubidium chloride and oxide,
(ii) caesium fluoride and sulfide? |
|
(b) |
Sodium reacts with
water to form hydrogen gas. Name three other elements you would expect to
react with water to form hydrogen. |
|
(c) |
Magnesium and calcium
form chlorides, MgCl2 and CaCl2. What compounds do you
expect fluorine, bromine and iodine to form with magnesium and calcium? |
|
(d) |
Fluorine forms with
carbon the compound carbon tetrafluoride, CF4. What compounds do
you expect chlorine and bromine to form with carbon? |
|
(e) |
Oxygen and nitrogen
with hydrogen form the compounds, water, H2O and NH3
respectively. Give the formulae you would expect for the compounds formed
between sulfur and hydrogen and between phosphorus and hydrogen.
|
| L5. |
Some properties of six
substances that are solids at room temperature are listed below. Which (if
any) of these would you consider to be a (a) metals
(b) ionic lattices (c) covalent molecular substances (d)
covalent lattices?
Give your reasons for each. |
| |
Melting
point (oC) |
Does
it conduct electricity? |
Other
properties |
| as
a solid |
as
a liquid |
| L |
63 |
yes |
yes |
soft
and malleable |
| M |
44 |
no |
no |
soft
and crumbly |
| N |
2990 |
no |
no |
extremely
hard |
| P |
2045 |
no |
yes |
very
hard |
| Q |
725 |
yes |
yes |
hard
but can be rolled into sheets |
| R |
373 |
no |
yes |
moderately
hard but can be ground into a powder |
For pages 70 and 75
| M1. |
1.00 g of a pale
blue solid was heated strongly in a crucible open to the atmosphere; it
changed to a black solid which weighed 0.64 g. After being allowed cool
down and stand on the bench for several hours, there was no further change
in the appearance or mass of the black solid. Explain why there has been a
decrease in mass. Is this a chemical or physical change? Why? Was the
original pale blue solid an element or a compound? Explain why.
|
| M2. |
(a) |
Classify the
italicised substances mentioned in the passage below as mixtures, elements
or compounds. In many cases the information in the passage will help you
with the classification. |
|
(b) |
Identify three
chemical and three physical changes in the passage and give your reasons
for so identifying them. |
|
|
Aluninium is
a substance in widespread use today in building materials, aircraft
construction and household utensils. Aluminium is obtained from bauxite
a red brown granular material composed of variable amounts of aluminium
oxide, iron oxide and silicaceous material (dirt).
The bauxite is ground up very finely then treated with hot concentrated sodium
hydroxide solution. This reacts with the aluminium oxide to form a solution
of sodium aluminate. The insoluble iron oxide and dirt are filtered
off and disposed of as red mud. Aluminium oxide is recovered by
cooling the sodium aluminate solution to precipitate out aluminium
hydroxide which is filtered off and heated to form aluminium oxide.
The pure white aluminium oxide known as alumina is then sent to an
aluminium smelter where an electric current is passed through a molten
mixture of alumina and cryolite a substance containing sodium,
aluminium and fluorine in fixed proportions. This process, called
electrolysis, breaks the aluminium oxide into aluminium and oxygen.
However rather than forming oxygen gas the electrolysis causes the oxygen
to combine with the graphite of the electrode to form carbon
dioxide.
The sodium aluminate mentioned above is also formed onaluminium utensils
cleaned in automatic dishwashers. Dishwashing powder contains
variable amounts of detergent, bleaches and sodium hydroxide. The
latter substance attacks aluminium to form the aluminate which discolours
the utensils.
|
For pages 79 and 82
| N1. |
Name
the following compounds: |
|
(a) SO3
(b) MgH2
(c) Li2S
(d) As2O3 |
(e) CF4
(f) Al2O3
(g) CuS
(h) NO2 |
(i) S2Cl2
(j) FeCl3
(k) Cl2O7
(l) Zn(OH)2
|
| N2. |
Write
the formulae of the following compounds: |
|
(a) |
(i)
(ii)
(iii)
(iv)
(v)
(vi) |
potassium oxide
dichlorine trioxide
antimony pentafluoride
aluminium hydroxide
silver oxide
iodine trichloride |
(vii)
(viii)
(ix)
(x)
(xi)
(xii) |
diphosphorus
trisulfide
iron(III) sulfide
silicon tetrabromide
iron(II) chloride
magnesium hydride
sulfur hexafluoride |
|
(b) |
(i)
(ii)
(iii) |
magnesium nitrate
silver carbonate
ammonium sulfate |
(iv)
(v)
(vi) |
iron(III) sulfate
aluminium phosphate
lead(IV) chloride
|
Answers to Exercises
| A1. |
If the air is clean
(just a mixture of gases) then it is homogeneous: if it contains dust
particles or water droplets (fog), it is heterogeneous. |
| A2. |
(a) |
Yes; solution of
sugar in water, honey, whiskey |
|
(b) |
No; by definition
(Table 1.1 page 6) a pure substance must be homogeneous. It is tempting to
think of a glass of water with ice in it as a pure substance, but by our
definition it is not because there are two phases present, liquid and
solid. |
| A3. |
Compound; because
its properties are different from those of the starting substances
(elements), it does not easily revert to the starting elements and it has
constant composition. |
| B1. |
Distillate, because
the distillate is richer in the more volatile component (the one with the
lower boiling point) which is ethanol (alcohol). Higher, same reason. |
| B2. |
Allow the two
liquids to settle and separate (they are immiscible, then use a separating
funnel (pages 15–6). |
| B3. |
A mixture; richer
in nitrogen (the one with the lower boiling point); fractionally distil
it (page 14), nitrogen |
| B4. |
Use a magnet: iron
attaches to it, lead does not. |
| B5. |
(a) |
By sublimation; put
the mixture in a flask fitted with a cooled test tube as in the photo on
page 68; the iodine sublimes on to the cold surface and the sand remains
on the bottom of the flask. |
|
(b) |
Again by
sublimation; this time the ammonium chloride vaporises and condenses on
the cooled surface. |
| B6. |
The milky mixture
resulting from adding sodium hydroxide solution to copper sulfate solution is heterogeneous,
because it can be separated into a solid and a liquid (solution) by
centrifuging; the two solutions reacted to form copper hydroxide, a pale
blue solid. The mixture of copper sulfate and ammonia solutions is a
true solution: it cannot be separated by centrifuging; the change in
colour results from a chemical reaction between the two solutions to form
the deep blue solution of a new compound called tetraamminecopper(II)
sulfate (or copper tetrammine sulfate). |
| B7. |
Add hexane to
dissolve the iodine, filter to separate the solids from the iodine in
hexane solution. Evaporate off the hexane to recover solid iodine. Then
sublime the carbon, ammonium chloride mixture as in answer B5.
Alternatively you could add water to the original mixture to dissolve the
ammonium chloride then after filtration separate the carbon and iodine by
sublimation.
Still another method would be to separate the iodine by dissolving it in
hexane and filtering it off, then adding water to the carbon, ammonium
chloride mixture to dissolve the latter and so separate that pair. |
| C1. |
(a) |
Oxygen, nitrogen
and hydrogen are gases, water, ethanol, ethyl acetate, ethylene glycol,
acetic acid, chloroform and hexane are liquids and the rest are solids. |
|
(b) |
(i) Water
and acetic acid would change to solids
(ii) Sodium and phosphorus would change from solid to liquid;
water, ethanol, ethyl acetate, chloroform and hexane would change from
liquid to gas (vapour). |
| D2. |
Determine its mass
then measure its volume by dropping it into a burette partly filled with
water and observing the change in volume (as in exercise D4). If it was
gold plated copper its density would be about 9 g/mL. If it was pure gold
(24 carat) its density would be 19.3 g/mL. If it was 18 or 9 carat gold
its density would be about 17 or 13 g/mL respectively. Density would
clearly distinguish between gold plated copper and any of the common gold
alloys. |
| E1. |
10% aspirin, 58%
sodium bicarbonate, 32% citric acid |
| E2. |
57% aggregate, 28%
sand, 13% cement. There has been a 1.5% loss of material during the
analysis so it is not as accurate as the measured masses suggest: hence
the rounding off in the percentages. |
| E3. |
There was some loss
of material as dust into the air or stuck to the seives, or even perhaps
some spillage. Some cement would have stayed with the aggregate and sand
(stuck on the surface of those coarse particles. This would have meant
that the percentage of cement was low. After separating the aggregate and
sand wash each with water to remove the cement, remove the
solution/suspension of cement in water by sedimentation and decantation
(page 13), dry the aggregate and sand then re-weigh each. This would give
more accurate measures of the amounts of aggregate and sand; take the true
mass of cement as the difference between starting mass and mass of
aggregate plus sand. |
| E6. |
The percentage
copper in the three samples is 58.7%, 49.0% and 56.1% (left to right).
Combined with the answer in exercise E5, we have clear evidence that the
composition of brass is variable. Therefore brass is a mixture (solid
solution) and not a compound. |
| F1. |
Because they do not
agree with the law of conservation of mass (matter) (page 69); mass of products
is 1.25 g compared with 1.36 g of starting material. |
| F2. |
1.43 g; law of
conservation of mass says that the mass of products must equal the mass of
reactant. |
| G1. |
(a) |
(i)
(ii)
(iii)
(iv) |
1 boron, 3 oxygen
and 3 hydrogen atoms
2 carbon, 4 hydrogen and 2 oxygen atoms
1 carbon, 4 hydrogen, 1 oxygen and 2 nitrogen atoms
6 carbon, 8 hydrogen, and 6 oxygen atoms |
|
(b) |
(i)
7 (ii) 8 (iii) 8 (iv) 24 |
| G2. |
(a) |
C2H2F4 |
|
(b) |
C3H7O2SN |
|
(c) |
C2H3O5N |
| H1. |
atomic numbers: (a)
14 (b) 33 (c) 18 (d) 12
mass numbers:(a) 29 (b) 75 (c) 40 (d) 24
Names and symbols: (a) phosphorus, P (b) arsenic, As (c)
argon, Ar
(d) magnesium, Mg |
| H2. |
Both have 12
electrons; same element (It is the number of protons that determines which
element an atom belongs to: as we shall see on page 141 these two atoms
are isotopes of the one element. |
| H3. |
Sr (2, 8, 18, 8, 2)
Zr (2, 8, 18, 10, 2) |
Tc (2, 8, 18, 13,
2)
Sb (2, 8, 18, 18, 5) |
Xe ( 2, 8, 18,
18,8) |
| H4. |
O (2, 6) S (2, 8,
6) Se (2, 8, 18, 6) Te (2, 8, 18, 18, 6);They all have six electrons in
their outermost energy level (that is, six valence electrons). |
| J1. |
9: (2, 7) Group 7
3: (2, 1) Group 1
12: (2, 8, 2) Group 2
18: (2, 8, 8) Group 0 (or 8)
15: (2, 8, 5) Group 5 |
4: (2, 2) Group 2
20: (2, 8, 8, 2) Group 2
30: (2, 8, 18, 2
13: (2, 8, 3) Group 3 |
36: (2, 8, 18, 8)
Group 0 (or 8)
6: (2, 4) Group 4
16: (2, 8, 6) Group 6 |
| J2. |
barium, 2
bromine, 7 |
gallium, 3
arsenic, 5 |
caesium, 1
selenium, 6 |
| J3. |
(a) |
K (2, 8, 8, 1); K+
(2, 8, 8) |
|
(b) |
F (2, 7); F–
(2, 8) |
|
(c) |
Al (2, 8, 3) ; Al3+
(2, 8) |
|
(d) |
S (2, 8, 6); S2–
(2, 8, 8) |
| J4. |
(a) |
(i) all are
(2, 8)
(ii) all are (2, 8, 8) |
|
(b) |
They all have a
stable noble gas configuration; because atoms tend towards the
configuration of the noble gas nearest to them. |
| K1. |
Your
diagram should show: |
|
(a) |
a lithium atom with
configuration (2, 1) donating an electron to a bromine atom (2, 8, 18, 7)
to form Li+ (2) and Br– (2, 8, 18, 8) |
|
(b) |
a magnesium atom
(2, 8, 2) donating 2 electrons to a sulfur atom
(2, 8, 6) to form Mg2+ (2, 8) and S2– (2, 8, 8) |
|
(c) |
each of two
sodium atoms (2, 8, 1) donating one electron to the same oxygen atom (2,
6) to form two Na+ (2, 8) ions and one O2– (2, 8)
ions |
| K2. |
(a) Sr2+
(2, 8, 18, 8) (b) I– (2, 8, 18, 18, 8) (c) Rb+
(2, 8, 18, 8)
(d) Se2– (2, 8, 18, 8) |
| K3. |
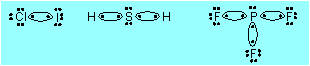
|
| K4. |
 |
| NaH, Na3N |
| K5. |
Ionic: a, c, e, g,
h
Mg2+, Ba2+, Na+, Ca2+, K+
(electron-dot diagrams show only valence electrons
(outer shell electrons): positive ions are formed by the atoms giving away
all the valence electrons, so their diagrams have no electrons on them.)
|
|
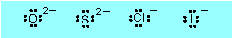
|
| K6. |
covalent:
b, d, f, i, j |
|
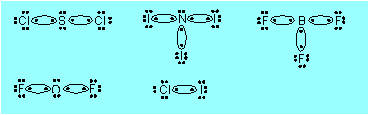 |
| L1. |
Arsenic tribromide
is a covalent molecular compound while magnesium bromide is ionic; ionic
compounds consist of an infinite lattice of positive and negative ions:
this is hard to break up so ionic compounds have high melting points.
However once molten there are mobile ions present that are able to move
under the influence of an electric voltage and so ionic compounds conduct
electricity.
Covalent molecular compounds consist of discrete small molecules with only
weak intermolecular forces between them; hence they melt at low
temperatures. These molecules are neutral so such substances do not
conduct electricity. |
| L2. |
It is a covalent
lattice |
| L3. |
(a) |
Ionic in SnCl2
and covalent in SnCl4; similar reasoning to that given in the
answer to exercise L1. |
|
(b) |
In SnCl2:
Sn2+ and Cl–
|
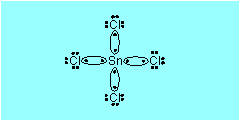 |
| L4. |
(a) |
(i) RbCl, Rb2O
(ii) CsF, Cs2S |
|
(b) |
Lithium, potassium,
rubidium and caesium |
|
(c) |
MgF2,
MgBr2, MgI2, CaF2, CaBr2, CaI2 |
|
(d) |
CCl4 and
CBr4 |
|
(e) |
H2S, PH3 |
| L5. |
(a |
L and Q; both are
conductors as solids and both are malleable |
|
(b) |
P and R; conduct
electricity as liquids but not as solids, relatively high melting points,
hard and/or brittle |
|
(c) |
M; low melting
point, does not conduct electricity either as a solid or as a liquid, soft |
|
(d) |
N; very high
melting point, does not conduct electricity, extremely hard |
| M1. |
A gas has been
formed; chemical, because two new substances have been formed (the black
solid and the invisible gas), and the change does not easily reverse. The
original solid was a compound, because it decomposed into two other
substances. |
| M2. |
(a) |
Mixtures: bauxite,
sodium hydroxide solution, solution of sodium aluminate, red mud,
dishwashing powder
Elements: aluminium, graphite (carbon)
Compounds: aluminium oxide, iron oxide, aluminium hydroxide, cryolite,
carbon dioxide, sodium hydroxide (as a solid) |
|
(b) |
Chemical: (i)
reaction of hot concentrated sodium hydroxide with aluminium oxide, (ii)
conversion of sodium aluminate to aluminium hydroxide, (iii) heating the
aluminium hydroxide to form aluminium oxide, (iv) electrolysis of alumina
to form aluminium, (v) reaction of oxygen with graphite to form carbon
dioxide, (vi) attack of sodium hydroxide on aluminium (in dishwashers)
Physical: (i) grinding up the bauxite, (ii) filtering off the red mud,
(iii) filtering off the aluminium hydroxide (iv) mixing alumina and
cryolite to form a solution (homogeneous molten mixture) |
| M3. |
(a) |
P2O5(s)
+ 3H2O(l) ® 2H3PO4(l) |
|
(b) |
4NH3(g)
+ 5O2(g) ® 4NO(g)
+ 6H2O(g) |
| M4. |
(a) |
Two atoms of solid
arsenic react with five diatomic molecules of liquid bromine to form two
molecules of solid arsenic pentabromide. |
|
(b) |
One molecule of
gaseous dichlorine heptoxide reacts with one molecule of liquid water to
form two molecules of liquid perchloric acid, each molecule of which
contains one hydrogen, one chlorine and four oxygen atoms. |
| N1. |
(a)sulfur
trioxide
(b) magnesium hydride
(c) lithium sulfide
(d) diarsenic trioxide (arsenic trioxide is acceptable)
(e) carbon tetrafluoride
(f) aluminium oxide |
(g) copper
sulfide (copper(II) sulfide is also acceptable)
(h) nitrogen dioxide
(i) disulfur dichloride
(j) iron(III) chloride
(k) dichlorine heptoxide
(l) zinc hydroxide |
| N2. |
(a) |
(i) |
K2O |
(v) |
Ag2O |
(ix) |
SiBr4 |
|
|
(ii) |
Cl2O3 |
(vi) |
ICl3 |
(x) |
FeCl2 |
|
|
(iii) |
SbF5 |
(vii) |
P2S3 |
(xi) |
MgH2 |
|
|
(iv) |
Al(OH)3 |
(viii) |
Fe2S3 |
(xii) |
SF6 |
|
(b) |
(i) |
Mg(NO3)2 |
(iii) |
(NH4)2SO4 |
(v) |
AlPO4 |
|
|
(ii) |
Ag2CO3 |
(iv) |
Fe2(SO4)3 |
(vi) |
PbCl4 |
260213 |


