|
Although Conquering Chemistry Preliminary Course (CCPC) fourth edition covers all the material in Module 1 of the current (2002) syllabus, it takes a slightly different approach in some places. CCPC begins similarly to the syllabus by introducing elements, compounds and mixtures (Syllabus Section 1) then shows that different parts of the Earth are made up of different mixtures and describing some important ones. Methods of separating mixtures are discussed (Sections 1.6 to 1.14). There is then a brief discussion of properties used to identify substances (Sections 1.15 to 1.19). Although this is not specifically mentioned in the syllabus, it is implicit in the early parts of the module. Gravimetric analysis of mixtures is then introduced (Section 1.20).The idea that there is a relationship between the reactivity of an element and its likely occurrence in the Earth as an uncombined element is discussed (Sections 1.21 and 1.22). Classification into metals and non-metals is introduced as is the Periodic Table, though the latter is not mentioned in the syllabus at this stage. That makes up Chapter 1. CCPC then combines Syllabus Sections 3 and 5 into Chapter 2 to present a more chemically coherent presentation of atomic structure and chemical bonding and its consequences for properties. After a brief review of atoms, symbols and formulae, the sequence followed is atomic structure (nucleus, electrons, protons and neutrons) (Sections 2.7 to 2.9), electron configuration (in simple form) (Sections 2.10 to 2.13), ionic bonding, covalent bonding, properties resulting from these bonding types, covalent network solids and metals (Sections 2.14 to 2.25). Syllabus Section 4, basically physical and chemical changes (reactions), is treated in the first part of Chapter 3 (Sections 3.1 to 3.6). The second part is a treatment of formulae and naming of simple inorganic compounds (Syllabus Sections 1, ninth dot point, and 4, sixth dot point). The way that CCPC relates to the HSC syllabus is shown in the Module 1 and the New South Wales HSC syllabus tables on pages 90–4. CCPC also includes significant amounts of revision of Stages 4 and 5 material (see below). For all modules CCPC attempts to cover all items in the Students learn column, to cover some items in the Students do column and to include exercises on as many of the other Students do items as possible. Exercises based on experiments are also common.
1.
Revision
This material is all treated in Conquering Chemistry so that if teachers feel the need to revise it, it is easily accessible, and if students need to look up the meaning of a basic terms or concepts, they can use this text. 2. Biosphere 3. How many naturally occurring elements? Actually there are several other elements such as francium and astatine which have not strictly been found in nature, but which are conceptually present on Earth. These are elements with only short-lived isotopes that are formed in the radioactive decay series of various naturally-occurring uranium and thorium isotopes. Because the U and Th isotopes are naturally present, and because in the laboratory we have identified their decay products, we must conclude that these decay products are present on Earth, even if in extremely small quantities. This question of how many naturally occurring elements raises an important
point about the methods of scientific discovery. Because we have not found
technetium and promethium, does this prove that they are not present on
Earth? Because you cannot find the needle in the haystack, does that prove it is
not there? Of course the short half lives of all the known isotopes of
promethium (less than 18 years) is added evidence for its non-occurrence
on Earth; however technetium does have a couple of quite long-lived isotopes (106
years). On balance the evidence for the non-occurrence of Tc and Pm on
Earth is strong, but not absolute. 1. Properties of elements and compounds (Section 1.2) Table 1.2 on page 7 contrasts the properties of a mixture, a compound and one of the elements making up the compound. Further contrasts between the properties of compounds and the elements that make them up are shown in the following tables.
Table
S1.2
(a) Copper is a pure substance. How do we show that it is an element? First it does not decompose when we heat it in the absence of air or when we pass an electric current through it. Secondly it undergoes a variety of chemical reactions with oxygen, chlorine, sulfur, nitric and sulfuric acids, silver nitrate solution, and so on. and in all of these reactions the copper-containing substance has a greater mass than the starting copper had. This shows that copper is an element, since it cannot be split directly or indirectly into two or more simpler substances. Similar arguments were used for all the elements to prove that they were, in fact, elements. (b) Lead nitrate is a white solid. It is soluble in water. It is homogeneous and its properties do not change after repeated purification procedures. Lead nitrate is therefore a pure substance. When lead nitrate is heated, a brown gas is evolved and a white solid remains. This solid is insoluble in water. Hence it is not just left-over lead nitrate. This new white solid always has a smaller mass than the sample of lead nitrate originally taken. Lead nitrate can therefore be decomposed into two other substances, a brown gas and another white solid. Lead nitrate is thus a compound. When heated it decomposes into nitrogen dioxide (the gas) and lead oxide, an insoluble white solid.
Silicon (‘con’ pronounced as in ‘concert’) is an element, symbol Si. It is widely used in the electronics industry. Its electrical properties depend greatly upon the presence of small amounts of impurities such as phosphorus, arsenic or boron. When suitably ‘doped’ with these elements, silicon can be used to make transistors, LEDs (light emitting diodes), photocells and computer chips. The major use of silicon as an element is in electronics components. Silicone (emphasis on ‘cone’ pronounced as in ‘ice cream cone’) is a compound. Or rather there is a whole family of compounds called silicones. These compounds contain the elements, silicon, oxygen, carbon and hydrogen. Silicones are characterised by having one or two carbon atoms attached to each silicon atom and by having silicon and oxygen atoms joined together in long chains such as –Si–O–Si–O–Si–O–Si–O–Si–O–.... Two- and three-dimensional
arrays can also be formed. There are hydrogen atoms attached to the carbon
atoms. The simplest silicone has the formula ((CH3)2SiO)n
Where n Silicones can be watery liquids (used for waterproofing fabrics), jelly-like liquids (used in the breast implants), Waxes (floor and car polishes) and rubbers (O-rings and seals in machinery). Silicone sealants sold in tubes in hardware stores contain a viscous liquid silicone which on exposure to water in the air undergoes further chemical reaction to form a firm rubbery substance which makes the actual seal. Some examples of these products are shown in the photo. Silicon and silicones dramatically illustrate the difference between elements and compounds. Unfortunately reading or listening to many people, we get the impression that they think the two words are just different ways of spelling or saying the same thing!
4. Other separation methods (Sections 1.6 to 1.13) Sections 1.6 to 1.13 treat the separation methods that are specifically mentioned in the syllabus. Some other methods that are widely used will now be described. (a) Centrifuging Paints are dispersions of small solid particles in liquids – water for water-based paints and hydrocarbons (petrol-like liquids) for oil paints. We would need to let paint stand for several weeks for the solids to settle to the bottom of the container. However if we centrifuge samples of paints, we can force the particles to settle out much more quickly. Blood is a dispersion of solids including red blood cells in an aqueous solution called plasma. We can separate the solid matter from the plasma by centrifuging. Centrifuging is widely used in industry to separate solids from liquids. Sometimes the centrifuge is designed to fling the water or solution away from the solids as in domestic clothes washing machines. In sugar mills and refineries crystalline sugar is separated from the syrup from which it formed in a similar way: the syrup is flung outwards through holes in the spinning drum to leave almost dry sugar crystals inside. (b) Coagulation and decanting (c) Magnetism Magnetic separations are widely used to separate magnetic materials (mainly iron and steel) from municipal garbage. (d) Sublimation Sublimation is the process in which a solid changes directly to a gas without passing through the liquid state (Table 1.6 on page 20). Sublimation can be used to separate mixtures such as ammonium chloride and sodium chloride (both soluble in water). (e) Paper chromatography The separation comes about because the different substances have different solubilities in the two liquids involved. One liquid is water trapped in the cellulose fibres of the paper (called the stationary phase) and the other is the liquid which moves up the paper (called the mobile phase). Substances with low solubility in the stationary phase and high solubility in the mobile phase move up the paper quickly. Those with high solubility in the stationary phase and low solubility in the mobile phase move slowly. Hence a separation occurs. There is a whole range of techniques for separating or analysing mixtures which go under the name of chromatography. Paper chromatography and other forms of chromatography are discussed in CCHSC Forensic Chemistry Option on pages 489-92 and 512-7; gas chromatography is briefly introduced in HSC Module 3 on pages196-7. 4.
The Periodic Table and electron configuration (Section 2.13) The first (extremely short) period
(hydrogen and helium) corresponds to the filling of the first energy level.
The fourth period (the first long period)
corresponds to first putting two electrons in the fourth level (K and Ca), then
completing the third level (scandium through to zinc), and finally semi-filling
the fourth level (to krypton 2, 8, 18, 8). Similar patterns apply for Periods 6 and 7. 5.
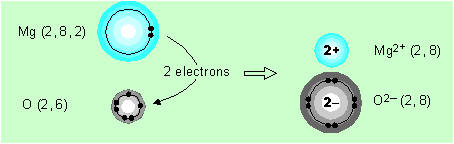
Another example of ionic bonding
(Section 2.15)
By losing two electrons, the neutral magnesium atom becomes the doubly charged positive ion, Mg2+. Similarly, by gaining two electrons, the neutral oxygen atom becomes the doubly charged negative ion, O2–, called the oxide ion. As each magnesium atom loses two electrons and each oxygen atom gains two electrons, the compound formed will consist of one oxygen atom per magnesium atom. Its formula will be MgO, which we may write as Mg2+O2– to emphasise the ionic bonding. Again there are no discrete molecules of MgO — just an infinite lattice of positive and negative ions very tightly bound together by electrostatic attraction. 6.
More everyday applications (Section 3.5, page 73)
230213 |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||