|
Sept/Oct
2002 Syllabus changes
The revised syllabus
and/or a version of the original syllabus with the changes marked in it can be
downloaded from the Board of studies web site (www.boardofstudies.nsw.edu.au).
Conquering Chemistry
is still a very close match to the revised syllabus though certain sections and
exercises, detailed below, can now be omitted. The first of the two sub-sections
below is for students: it tells you what you can omit from CCPC. The
second sub-section is primarily for teachers: it gives more detail about what
the changes are and how they affect CCPC.
In both
sub-sections LC, MC and RC mean left, middle and right column respectively and
DP means dot point. The references are to the original syllabus (1999 or
March/April 2001)
1.
For students
As a
result of these syllabus changes you should omit the following:
|
In
Chapter 1
| · |
You could omit
Section 1.5 on page 12, though it is worth reading anyway. However you
will not be expected to recall information in Table 1.4. |
| · |
Omit Sections 1.21,
1.22 and 1.23 on pages 27 to 31.
Omit Exercises 27, 28 and 29. |
| · |
You could omit
Section 1.27 on pages 34 and 35 though it is still a good place to
introduce the Periodic Table. You should not omit Physical states of
the elements on page 36. |
In
Chapter 2
| · |
Although there have
been some syllabus changes that affect Chapter 2, I believe that you
should not omit any sections of this chapter |
In
Chapter 3
| · |
No omissions from
this chapter. |
In
Revision Test for Module 1
Omit Questions
4, 6, 13 and 20. Allow 70 minutes for the
test and mark it out of 39.
|
2.
For teachers
The
changes to the syllabus and their consequences for Conquering Chemistry
are set out in the following table.
| Syllabus
change |
Consequence
for using CCPC |
Section 8.2.1
In MC
Delete DP 2
Delete DP 4
Delete DP 5
Change DP 9
In RC
Insert new DP 1
Partially delete old DP 2
|
None
Omit Section 1.5
Omit Section 1.23; omit Exercise 29 on page 32
None
None
None |
Section 8.2.2
In MC
Delete DP 1
Delete DP 2
Delete DP 6 |
Omit Sections 1.21 and 1.22; omit Exercises 27 and 28 on page 32
None
Could omit Section 1.27 though this is a good place for a first
introduction to the Periodic Table. |
Section 8.2.3
In MC
Delete DP 2
Insert new DP 3 (after old DP 3)
Other changes and the one in RC are 'editorial' rather than substantive
|
Puzzling deletion: students still need to know the basics of atomic
structure to understand other syllabus topics
Treated in Section 2.9
None
|
Section 8.2.4
In MC
Delete DP 3, 6 and 7
In RC delete DP 4 |
None
None
|
Section 8.2.5
In MC
Delete DP 2
Delete DP 3
Delete old DP 8
Insert new DP 8
|
None (At first sight this may suggest that Table 1.2 on page 7-8 can be
omitted, but really that table is there to illustrate mixtures, compounds
and elements so still is needed.)
Was delayed until Chapter 5 in CCPC
At first sight could omit Section 2.21 and related part of Table 2.6, but covalent
lattices are required in RC DP 2 (and for graphite and diamond in Module 3) so
this material is still required.
Already in CCPC (bottom of page 60)
|
The
(original) syllabus and the book
Although Conquering Chemistry Preliminary
Course (CCPC) covers all the material in
Module 1 of the original syllabus, it takes a slightly different approach.
CCPC begins similarly to the syllabus by
starting (Syllabus Section 1) with the idea that different parts of the Earth
are made up of different mixtures, describing some important ones, and
discussing separation of mixtures (including some gravimetric analysis of
mixtures). It then (Syllabus Section 2) looks at the five most abundant elements
in the different 'spheres' of the Earth and at the forms in which they occur
(Sections 1.21 to 1.23 which now can be omitted),
introducing the ideas of constancy of elemental composition and that only
non-reactive elements occur in uncombined states (Sections 1.24 and 1.25, still
in the syllabus). Classification into metals and
non-metals is introduced as is the Periodic Table. That makes up Chapter 1.
CCPC then combines Syllabus Sections 3 and
5 into Chapter 2 to present a more chemically coherent presentation of atomic
structure and chemical bonding and its consequences for properties.
Syllabus Section 4, basically physical and
chemical changes (reactions), is treated in the first part of Chapter 3. The
second part is a treatment of formulae and naming of simple inorganic compounds
(Syllabus Sections 1, ninth dot point, and 4, sixth dot point).
CCPC also includes significant amounts of
revision of Stages 4 and 5 material (see below).
For all modules CCPC attempts to cover all
items in the Students learn column, to cover some items in the Students
do column and to include exercises on as many of the other Students do
items as possible. Exercises based on experiments are also common.
For a more detailed comparison of CCPC
with the syllabus click on
Charts relating C.C. sections to syllabus items.
These tables show the Students learn
column of the syllabus (in abbreviated form) along with the sections of CCPC
that treat individual items.
Some comments
1.
Revision
At first sight Module 1 in C.C. appears
very long (100 pages compared with an average of 80 per module in the
Preliminary Course). This is mainly because the book revises a lot of material
that the syllabus takes as assumed knowledge, namely
-
mixtures,
compounds and elements
-
atoms
and molecules
-
some
relationships between elements in the Periodic Table
-
particle theory of matter
-
atomic
structure (nucleus, electron cloud, protons, neutrons)
-
word
equations and qualitative descriptions of reactants and products in
decomposition reactions
-
common
names and formulae for common compounds
This material is all treated in Conquering
Chemistry so that if teachers feel the need to revise it, it is easily
accessible, and if students need to look up the meaning of a basic terms or
concepts, they can use this text.
2. Biosphere
There may be a problem with the word biosphere.
All standard texts biological, geological, chemical define it as the
portion of the Earth inhabited by or used by living matter (and so it is the
hydrosphere, atmosphere and part of the lithosphere). See page 10. However from the contexts
in which the word is used in the syllabus, the meaning appears to be living
matter. It is perhaps necessary to treat both the proper biosphere and
living matter (that is what C.C. does on pages 27 to 30).
3. How many naturally occurring elements?
Page 27 claims that there are 92
naturally occurring elements. I have been told that this is incorrect.
Apparently the 'official' answer to a question in a past HSC paper was that
there are 89 atomic numbers 1 to 92, but excluding technetium (At No 43),
promethium (61) and astatine (210). The CRC Handbook of Physics and
Chemistry claims that there are 91 atomic numbers 1 to 94 less technetium
and promethium (and presumably another one). That source claims that both neptunium (At No 93) and plutonium
(94) have been found in trace amounts in certain uranium-containing rocks. They
are believed to have been formed by reaction of neutrons with uranium in natural
transmutation processes.
Actually there are several other elements such as francium and astatine which
have not strictly been found in nature, but which are conceptually present on
Earth. These are elements with only short-lived isotopes that are formed in the
radioactive decay series of various naturally-occurring uranium and thorium
isotopes. Because the U and Th isotopes are naturally present, and because in
the laboratory we have identified their decay products, we must conclude that
these decay products are present on Earth, even if in extremely small
quantities. One radioactive decay series is discussed on page 72 of Conquering
Chemistry HSC Course.
This question of how many naturally occurring elements raises an important
point about the methods of scientific discovery. Because we have not found
technetium and promethium, does this prove that they are not present on
Earth? Because you cannot find the needle in the haystack, does that prove it is
not there? Of course the short half lives of all the known isotopes of
promethium (less than 18 years) is added evidence for its non-occurrence
on Earth; however technetium does have a couple of quite long-lived isotopes (106
years). On balance the evidence for the non-occurrence of Tc and Pm on
Earth is strong, but not absolute.
Supplementary material
1. Properties of elements and compounds
(Section 1.2)
Table 1.2 on pages 7-8
contrasts the properties of a mixture, a compound and one of the elements making
up the compound.
Further contrasts between the properties of compounds and the elements that
make them up are shown in the following tables.
Table S1.1
Properties of the compound sodium chloride and
the elements forming it
| Sodium |
Chlorine |
Sodium
chloride |
| lustrous silvery
solid |
pale yellow-green
gas |
white crystalline
solid |
| soft and pliable |
|
hard and brittle |
| melts at 98oC |
condenses to
liquid at 35oC |
melts at 800oC |
| conducts
electricity |
|
solid does not
conduct electricity |
| combines rapidly
with atmospheric oxygen |
unaffected by air |
unaffected by air |
| violent chemical
reaction with water |
dissolves
slightly in water |
readily dissolves
in water; solution conducts electricity |
| reacts chemically
with chlorine, sulfur, phosphorus |
reacts chemically
with aluminium, zinc, copper |
does not react
with any elements |
Table
S1.2
Properties of the compound carbon disulfide and
the elements forming it
| Carbon
(graphite) |
Sulfur |
Carbon
disulfide |
| black powdery
solid |
yellow solid |
colourless liquid |
| odourless |
odourless |
unpleasant odour |
| melts at 3727oC |
melts at 113oC |
boils at 46oC
melts (freezes) at 111oC
|
| conducts
electricity |
does not conduct
electricity |
does not conduct
electricity |
| burns in air |
burns in air |
does not burn in
air |
| insoluble in
hexane |
insoluble in
hexane |
soluble in hexane |
Table
S1.3
Properties of haematite (iron(III) oxide) and the elements that make it up
| Iron(III)
oxide, Fe2O3
haematite |
Iron, Fe |
Oxygen,
O2 |
| red powder
(solid)a |
shiny grey solid |
colourless gas |
| melting point
1565oC |
melting point
1535oC |
boiling point
183oC |
| density 5.2 g/mL |
density 7.9 g/mL |
|
| does not conduct
electricity; poor conductor of heat |
good conductor of
electricity and heat |
does not conduct
electricity; poor conductor of heat |
| hard and brittle |
malleable and
ductile |
|
| can be converted
to a simpler substance, iron by heating with carbon so is a compound |
cannot be
decomposed into simpler substances so is an element |
cannot be
decomposed into simpler substances so is an element |
| fairly unreactive |
burns when heated
in oxygen to form Fe2O3; rusts in moist air
|
reacts with many
elements, both metals and non-metals |
| a
often mined as a brown-black rock |
2. Other examples of elements, compounds
and mixtures
(to supplement the example on pages
8(bottom)9)
(b) Copper is a pure substance. How do we
show that it is an element? First it does not decompose when we heat it in the
absence of air or when we pass an electric current through it. Secondly it
undergoes a variety of chemical reactions with oxygen, chlorine, sulfur, nitric
and sulfuric acids, silver nitrate solution, and so on. And in all of
these reactions the copper-containing substance has a greater mass than the
starting copper had. This shows that copper is an element, since it cannot be
split directly or indirectly into two or more simpler substances.
Similar arguments were used for all the elements
to prove that they were, in fact, elements.
(c) Lead nitrate is a white solid. It is
soluble in water. It is homogeneous and its properties do not change after
repeated purification procedures. Lead nitrate is therefore a pure substance.
When lead nitrate is heated, a brown gas is evolved and a white solid remains.
This solid is insoluble in water. Hence it is not just left-over lead nitrate.
This new white solid always has a smaller mass than the sample of lead nitrate
originally taken. Lead nitrate can therefore be decomposed into two other
substances, a brown gas and another white solid. Lead nitrate is thus a compound.
When heated it decomposes into nitrogen dioxide (the gas) and lead oxide, an
insoluble white solid.
3.
Other separation methods (Sections 1.6
to 1.13)
Sections 1.6 to 1.13 treat the separation methods
that are specifically mentioned in the syllabus. Some other methods that are
widely used will now be described.
(a) Centrifuging
Sedimentation occurs quickly if the solid
particles are relatively big or dense. For smaller particles it can take an
inconveniently long time. Sedimentation can be speeded up by centrifuging the
mixture. This means putting the mixture in a suitable container and spinning it
so that the solid particles get subjected to centrifugal forces which are much
stronger than the force of gravity. This pushes the solid particles outwards and
so away from the liquid. The machine that does this is called a centrifuge.
In a laboratory centrifuge the mixture is placed in a large test tube which is
then spun so that the solids are forced to the bottom of the tube; the clear
liquid can then be decanted or sucked off. The photo below shows a laboratory
centrifuge and a sample before and after centrifuging: it comes from Conquering
Chemistry HSC Course (CCHSC), page 201.

Paints are dispersions of small solid particles
in liquids water for water-based paints and hydrocarbons (petrol-like
liquids) for oil paints. We would need to let paint stand for several weeks for
the solids to settle to the bottom of the container. However if we centrifuge
samples of paints, we can force the particles to settle out much more quickly.
Blood is a dispersion of solids including red blood cells in an aqueous solution
called plasma. We can separate the solid matter from the plasma by centrifuging.
Centrifuging is widely used in industry to
separate solids from liquids. Sometimes the centrifuge is designed to fling the
water or solution away from the solids as in domestic clothes washing machines.
In sugar mills and refineries crystalline sugar is separated from the syrup from
which it formed in a similar way: the syrup is flung outwards through holes in
the spinning drum to leave almost dry sugar crystals inside.
(b) Coagulation and decanting
When a suspension of very fine particles in water
is boiled, the particles often collide with each other with such force (at the
higher temperature) that they stick together and form much bigger (heavier)
particles. These bigger particles then more readily settle to the bottom of the
container and so allow the clear liquid or solution to be decanted off. This
process of small particles combining to form bigger ones is called coagulation.
A combination of coagulation and decanting (page 13) is commonly used to obtain better
quality drinking water on extended wilderness camping trips: muddy water is
boiled for half an hour or so (to coagulate the particles) then let stand
overnight. By morning the clay (mud) has settled to the bottom and the clear
water can be decanted off for drinking and cooking.
(c) Magnetism
If one substance in a mixture is magnetic while
the others are not, then we can separate out the magnetic substance with a
magnet. A mixture of iron filings and sulfur can be separated in this way.
Magnetic separations are widely used to
separate magnetic materials (mainly iron and steel) from municipal garbage.
(d) Sublimation
While most substances pass from solid to liquid
to gas, there are some such as dry ice (solid carbon dioxide), iodine and
ammonium chloride which change directly from solid to gas. This is called sublimation.
If some iodine crystals are placed in a conical flask with a test tube
containing ice water suspended in it as in photo on page 78 and gently heated,
purple vapour will be seen to form from the crystals and it will condense back
to crystals on the cool surface of the test tube. This is sublimation followed
by condensation back to solid.
Sublimation is the process in which a
solid changes directly to a gas without passing through the liquid state.
Sublimation can be used to separate mixtures such
as ammonium chloride and sodium chloride (both soluble in water).
(e) Paper chromatography
Paper chromatography is a technique for
separating mixtures. Separation occurs because the substances to be separated
have different solubilities in two solvents. In school laboratories paper
chromatography is often used to separate the components of various inks. A spot
of ink is placed on a strip of filter paper which is then suspended in a
suitable liquid. As the liquid creeps up the paper (by capillary attraction), it
washes the components of the ink upwards at different rates. After a few
minutes different-coloured, separated spots can be seen as is shown in the
diagram below (from page 477 of CCHSC).
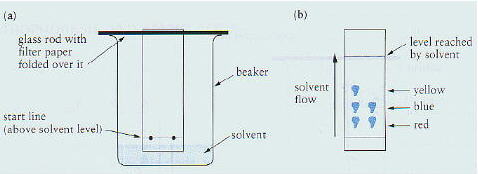
The separation comes
about because the different substances have different solubilities in the two
liquids involved. One liquid is water trapped in the cellulose fibres of the
paper (called the stationary phase) and the other is the liquid which
moves up the paper (called the mobile phase). Substances with low
solubility in the stationary phase and high solubility in the mobile phase move
up the paper quickly. Those with high solubility in the stationary phase and low
solubility in the mobile phase move slowly. Hence a separation occurs.
There is a whole range of techniques for
separating or analysing mixtures which go under the name of chromatography.
Paper chromatography is discussed further in CCHSC on pages 476-8
with gas chromatography briefly introduced on page 189 (of that book).
4.
The Periodic Table and electron configuration (Section 2.13)
It is instructive to see how the Periodic Table
relates to the filling of electron energy levels. We can use Figure 2.9(b) on
page 53 to
obtain the order in which energy levels are filled, and by working through the
Periodic Table (see inside front cover) we get the schematic table shown below.
The first (extremely short) period
(hydrogen and helium) corresponds to the filling of the first energy level.
The second period corresponds to filling
the second energy level (Li to Ne). The big horizontal gap has been left between
Be and Al because in later periods we have to fit in extra elements.
The third period (Na to Ar) corresponds to semi-filling (going up to 8 electrons) the third energy level; this takes us to
the stable argon configuration, 2, 8, 8.

The fourth period (the first long period)
corresponds to first putting two electrons in the fourth level (K and Ca), then
completing the third level (scandium through to zinc), and finally semi-filling
the fourth level (to krypton 2, 8, 18, 8).
The fifth period is formed by putting two
electrons into the fifth level (Rb abd Sr), then building the fourth level from
8 to 18 in what is another transition series (yttrium to cadmium), and finally
semi-filling the fifth level to 8, ending with xenon (2, 8, 18, 18, 8).
Similar patterns apply for Periods 6 and 7.
5.
Another example of ionic bonding
(Section 2.15)
Magnesium and oxygen combine to form magnesium
oxide.
Magnesium, with electron configuration (2, 8, 2),
(Table 2.4), loses two electrons to become like neon (2, 8). Oxygen (2, 6) gains
two electrons, also to become like neon.
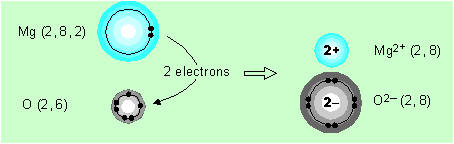
By losing two electrons, the neutral magnesium
atom becomes the doubly charged positive ion, Mg2+.
Similarly, by gaining two electrons, the neutral oxygen atom becomes the doubly
charged negative ion, O2,
called the oxide ion.
As each magnesium atom loses two electrons and
each oxygen atom gains two electrons, the compound formed will consist of one
oxygen atom per magnesium atom. Its formula will be MgO, which we may write as
Mg2+O2
to emphasise the ionic bonding. Again there are no discrete molecules of MgO
just
an infinite lattice of positive and negative ions very tightly bound together by
electrostatic attraction.
6.
More everyday applications (Section 3.5, page 85)
Several examples of the
use of decomposition and direct combination reactions in everyday life are given
in Section 3.5. Some additional ones are:
- sodium chloride solution is electrolysed to
form chlorine and sodium hydroxide, two very important industrial chemicals
(to be discussed in CCHSC Chapter 10); this same electrolysis is used
in some home swimming pools for chlorination (sanitisation) of pool water
- in some industries water is electrolysed as a
source of hydrogen gas (though this is not a major industrial source of
hydrogen)
- decomposition of silver salts particularly
silver bromide by light is the
basis of photography
- ultraviolet light is used to decompose certain
molecules in bacteria in some commercial sterilisers.
- the burning of sulfur (combination with
oxygen) is used industrially to make sulfur dioxide which is then used to
make sulfuric acid, the most widely used industrial chemical
|

