| p. 29 line
5: |
separates (one t only) |
| p. 40
caption to Fig 4.8: |
nanometres (n not m) |
| p. 50 last
line of text: |
CaF2 (subscript
2) |
| p.52 4th
line of example 3: |
Cl2 (subscript
2) |
| p. 59
exercise 31: |
should
be an asterisk in front of the 31
(i.e. no answer given) |
| p. 63
exercise 33: |
should
be an asterisk in front of the 33
(i.e. no answer given) |
| p. 66
question 46: |
The
headings for the 4th and 5th columns should be set out as:
Electrical conductance
Pure
In aq. soln
That is, electrical conductance refers to both columns |
| p. 76 4th
line from bottom: |
Mg2+
(superscript 2+) |
| p. 77 Table
3.2, 4th row: |
delete
H– |
p. 90
right-hand column of
table, 6th row: |
change
1.12 to 1.13 and 1.14 to 1.15 |
|
next row: |
change
1.13 to 1.14 |
|
next row: |
change
1.19 to 1.20 |
|
bottom row: |
change
1.20, 1.21 to 1.21, 1.22 |
| p. 91 1st
row after heading: |
change
1.22 to 1.23 |
|
next row: |
delete 1.23 |
| p.102
bottom row of table: |
'for
both balance is roughly equal amounts of silver and gold' should
be in the second (composition) column, under '37.5% gold' |
| p.
113 equation (4.2) |
Should be
just Cu2S (not 2Cu2S) |
p. 139 in
the yellow-green
shading: |
AwByCz should be AwByCz
(w, y, z subscripts) both in the 1st line and in the bottom
line of Equation 5.3 |
p. 143 5th
line (in the
equation): |
change
2H2(l) to 2H2O(l) (i.e. insert O |
| 8th, 9th,
10th, 11th lines: |
change O2
to CO2 (four
times) |
| p. 189 5th
line after Fig 7.3: |
change
6.4 to 6.5 |
p. 191 Fig
7.6, in the text at
the top, after Od–: |
insert
'or Nd–' |
p. 199
Table 7.5 4th blue
row: |
line
up '·
a few soluble' with '·
starch, glycogen, enzymes' |
| p. 205
Table 8.1 bottom line: |
'(except
Groups 1, 2 and NH4+)' should line up with
'·
sulfides' |
| p. 214 5th
line Example 6 c: |
4th
line
106 not 106 |
| p. 219
Example 11 4th line: |
in
the chemical equation ´
should be + |
| p. 220 4th
line: |
delete –1
(i.e. g/mol) |
p. 252 set
of dot and ?
structures in the
middle
of the page: |
There are 4
structures missing on the left-hand side of this diagram, the
all-black-dot ones:
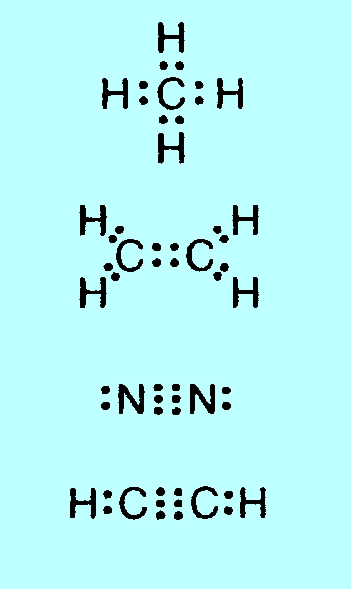 |
| p. 264 5th
line: |
Table
9.9 (not Figure
9.9) |
| p. 265 5th
line: |
insert
'compound' after 'straight-chain'
(a straight-chain compound with five carbon?) |
p. 267
Figure 9.11, bottom
left-hand
shaded bit: |
first
CH2 should be CH3 |
| p. 299 7th
line: |
103 to 106
(not 103 to 106 ) |
p. 314
right-hand column
Ans 14 3rd line: |
Ca not Sr |
|
Ans 15 1st line: |
underline
3 and 6 (B 2, 3;
O 2. 6 ) |
|
2nd line: |
underline
last 2 (Ca
2, 8, 8, 2 ) |
|
3rd line: |
underline
2 in Mn and 6 in Se (Mn
2, 8, 13, 2; Se 2, 8, 18, 6
) |
|
4th line: |
underline
final 1 (Rb 2,
8, 18, 8, 1 ) |
| p. 315 Ans
11 d: |
delete
(h) (d CaH2
) |
|
Ans 18 a: |
dinitrogen
monoxide (not dinitrogen oxide) |
| p. 327 Ans
14 b: |
Mg(NO3)2.6H2O
(not MgSO4.6H2O) |
| p. 328 Ans
16 a: |
change
2.0 to 3.2 (3.2´
10–3 g ) |
| Ans
17: at end of line: |
insert
C8H16 |
| p. 334 Ans
8: |
Figure
10.1(b) (not
10.21(b) ) |
| p. 341 in
index |
insert
'solutions' after 'apparatus for measuring' |
