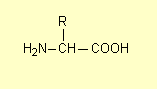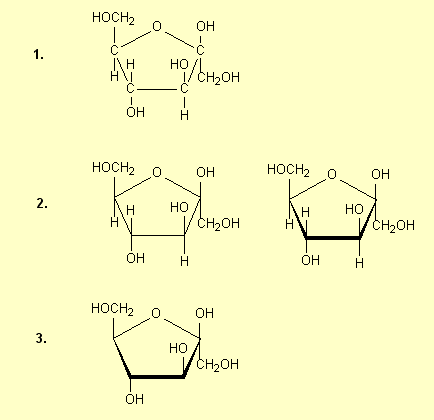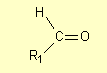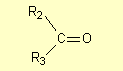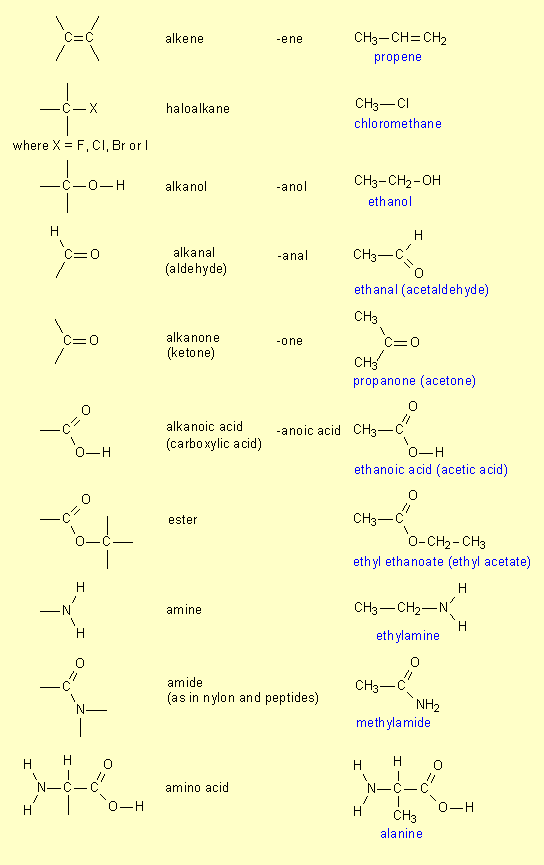|
Choosing an option
Two major considerations for teachers in selecting the one option (of the five
available) for their students to study, are first their students' likely interest in the topic and
secondly their ability to cope with
the amount and complexity of the material. Other considerations are likely to be
how well the material integrates with and reinforces core material and how
straight-forward and/or predictable are likely examination questions on the
material. The availability of equipment for laboratory work is another
consideration.
Judging by the number of pages I needed to cover
the material in each of the three options treated in Conquering Chemistry,
the Forensic chemistry option would appear to contain the most material
with much of it being quite complex for
students whose background in organic chemistry is just the core material. While
a good option for students with a strong biological interest, it could be
daunting for weaker students. The name of this option is somewhat deceptive:
while there are frequent attempts to relate the material to what
forensic scientists do, basically this option is an introduction to
biochemistry (or biological chemistry as it used to be known in the
pre-2000 syllabus). If one were genuinely after an introduction to
forensic chemistry, such large slabs of the chemistry of sugars and
proteins would not be included. The name is considerably more attractive
to students than the material it contains, so teachers should not rush
into selecting this option before having a good look at its
contents.
This option treats some fairly
sophisticated instrumental techniques – electrophoresis, gas
chromatography, HPLC, mass spectrometry and atomic emission
spectroscopy. Teachers need to know that they will at least be able to
show examples of these instruments to their students before adopting
this option. Having a university or technical college nearby with a
welcoming chemistry department would be a big advantage. While this
would be adequate for most instrumental techniques, the school really
needs to have at least very basic electrophoresis equipment to do this
option. Simple chromatography experiments present no problem for the
typical school lab. Also note that although the syllabus says emission
spectra can be studied either with flame tests or a hand spectroscope,
the spectroscope is essential, because students have to examine the
spectrum of mercury and that cannot be done with a flame test (mercury
is too poisonous).
Despite these difficulties, this option
has been moderately popular and students who have done it have often
been quite enthusiastic about it.
With the current heavy emphasis on factual recall
in exam questions, the quantity of material to be assimilated is probably a key
consideration in selecting an option.
In writing Conquering Chemistry I thought
that the other two options, Biochemistry of movement and Chemistry of
art, contained too much material of too diverse a nature for the seven weeks
available for the study of the option. Of course these options will particularly
appeal to certain groups of students – those strongly interested in sport and
dance and those with a strong artistic bent – and strong motivation can easily
overcome barriers that may seem daunting to others.
Some general comments
1. Exam questions
HSC exam questions in
the Options section of the paper differ somewhat from questions in the core
section in that:
| · |
there is more emphasis on
recall of learned information about the context rather than on the underlying
chemical principles |
| · |
there is always at least one
question about a compulsory experiments often with a bit about risk
assessment |
| · |
there is always at least one
extended response question (question with five or more marks and without
detailed instructions) and it is usually is of the discuss, analyse, assess, evaluate type, and |
| · |
problem-solving or calculation
type questions are rare (though in this option over the period 2003-8
there has been a simple problem on DNA analysis and two problems on
HPLC. |
Because of this absence of calculations and
problems from the options sections of the HSC exam paper, there are no Further Exercises sections on the Options
pages of this website.
2. Structural formulae
Occasionally the HSC exam question has instructed students to draw the structural formula of a compound (or class of
compound). Strictly speaking a structural formula shows all the chemical bonds
as for example the general structure of an amino acid is (2007):
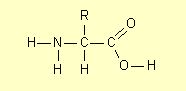
where R is a hydrogen atom or a carbon-containing group (such as an alkyl
group).
Reports from the examination centres have not made it clear whether condensed structural formulae
such as
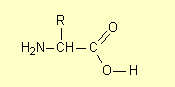 or
or 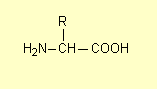
would get full marks, though in the absence of a comment to the contrary they
probably do. Students should be encouraged to draw complete structures (unless the
molecule is fairly complex) and certainly they should avoid abbreviating
carboxylic acid and ester groups to –COOH and –COO–.
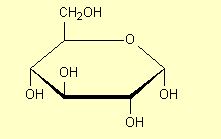
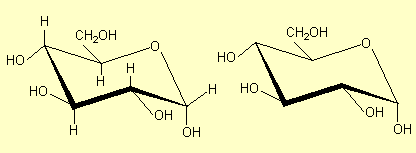
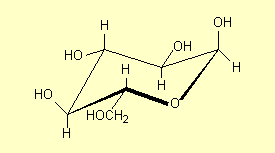
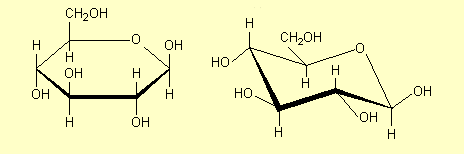
3. Structures for saccharides
Because saccharides are fairly complex molecules,
a variety of structures can be drawn for the one compound and it is essential
that students can interpret any given structure and recognise the compound
in question. For example for glucose some of the structures that are commonly
drawn are:
| 1. |
Draw the
hexagonal ring flat with the H and OH groups above and below the
ring and show all atoms |
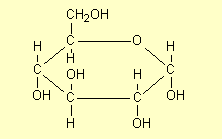 |
| 2. |
Omit the
C atoms. This is a common practice for chemists: at every apex
in the structure there is a C atom. The planar ring here and in
1 is drawn perpendicular to the plane of the paper; sometimes
the 'front' bonds are drawn heavier than the others to denote
this. |
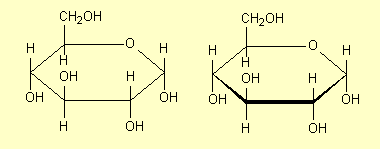 |
| 3. |
Omit the
H atoms also; this is another common practice, though not
recommended for HSC students. At each apex
there is a C atom which has enough H atoms on it to bring the
valence up to four. Double and triple bonds, if present,
are always drawn. |
 |
| 4. |
Draw the ring in its
true shape which is puckered – a chair form is what chemists
call this – and again the C and H atoms can be shown or just
'understood' to be present. |
 |
| 5. |
And with any one of
these the whole structure can be tipped upside down (rotated
about an axis through the extreme left- and right-hand C atoms).
We had to do this to alternate glucose units in cellulose in Fig
13.6 on p 470-1 though there it was the b
isomer involved (not the a
one as in all these structures here). |
 |
| 6. |
Or the
structure in 4 can be rotated
about an axis perpendicular to the plane of the paper. |
 |
All of these structures are a-glucose
– the carbonyl OH is on the opposite side of the ring from the CH2OH.
The carbonyl OH is the one at the right hand end of the structures (except in
Structure 6). See p 468 and 474. b-glucose
has the H and OH on the carbonyl carbon interchanged (p 474-5):
You may be required to draw a structure of glucose. The following rule may help
you memorise the structure of glucose: starting from the O atom and going to the
C with CH2OH attached and keeping going around the ring in that
direction, the OH groups are opp, same, opp, opp for a
and opp, same, opp, same for b.
Opp means on the opposite side of the ring from the CH2OH
group, and same means on the same side of the ring. Structures 2 above
are the easiest to see this on, but the rule applies to all the structures
drawn.
For fructose there is less variety in the
structures that can be drawn because the five-membered ring is virtually planar.
For 1, 2 and 3 above we have for b-fructose
(the form used to make sucrose):
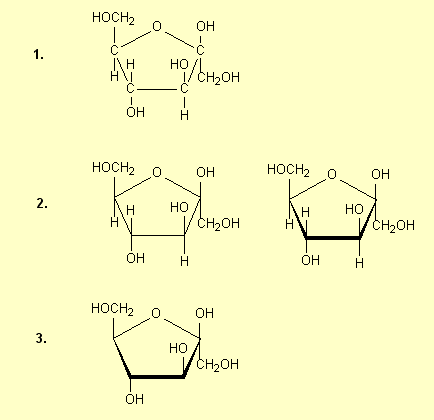
If we draw sucrose with glucose on the left (as is commonly done) we need to
rotate our fructose molecule 180o around an axis perpendicular to the
plane of the paper through the O atom. Rotating the right-hand structure of 2
gives:
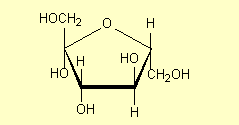
(The structure of sucrose is given on p 468-9.)
a-fructose
(in the style of 2) is:
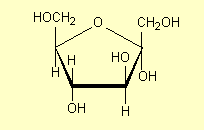
4. Carbonyl compounds,
a-b
isomerism and disaccharides
(This account
contains more detail than is required for the HSC but it may help you understand
better what is going on in the test for reducing and non-reducing sugars.)
Carbonyl compounds are compounds
that contain the carbonyl group C=O. Two classes of carbonyl compound are aldehydes
and ketones. Aldehydes have the general structure
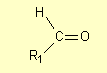
where R1 is a hydrogen atom or a carbon-containing group (such
as an alkyl group). Ketones have the general structure
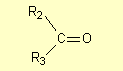
where R2 and R3 are carbon-containing groups; R2
and R3 cannot be hydrogen atoms but they can be the same group (e.g.
methyl groups).
One property of these carbonyl compounds is that they react reversibly with
alcohols to form hemi-acetals and acetals:
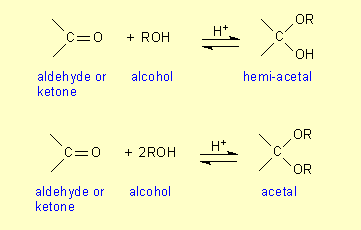
The ring structures of simple monosaccharides are actually hemi-acetals (see Fig
13.10 on p 474). Rather than the carbonyl and alcohol groups being on separate
molecules as in the above equations, they are both on the same molecule. When
two monosaccharides combine together to form a disaccharide they are actually
forming an acetal: one monosaccharide is acting as a hemi-acetal while the other
is the alcohol. In Equation 13.1 on p 468 the hemi-acetal glucose
(right-hand glucose) forms an acetal with an alcohol group of the left-hand one
and the maltose molecule formed is an acetal.
In carbohydrate contexts an acetal is called a glycoside and the
monosaccharide–O–monosaccharide linkage is called a glycosidic bond
or glycosidic link. For
example the disaccharides, sucrose, maltose and galactose, are glycosides or
contain glycosidic bonds.
The hemi-acetal form of a monosaccharide can easily
convert back to the carbonyl form, that is convert to the open-chain form of the
monosaccharide as in Fig 13.10 on p 474. This is how a-b
isomerism occurs and is why an aqueous solution of a monosaccharide contains
both isomers. However once a glycoside has formed with an alcohol or another
monosaccharide, the compound no longer easily reverts to a hemi-acetal; that is
the glycosidic link is not easily broken. Hence sucrose does not easily revert
to glucose and fructose; it needs an acid or enzyme catalyst to do it. And this
is why sucrose is a non-reducing sugar (p 475-6): there is no carbonyl group to
reduce Ag+ or Cu2+.
Actually it is not strictly correct to say that
carbonyl groups reduce Ag+ or Cu2+ . Certainly aldehydes
do but normal ketones do not. In fact the Benedict's and Fehling's solutions
tests are used to distinguish between aldehydes and ketones. However if there is
a CH2OH group beside the C=O group, then that combination can reduce
Ag+ or Cu2+ as shown on p 475. If you are not convinced
that going from –CO–CH2OH to –CHOH–COOH is oxidation then
write the half reaction:
–CO–CH2OH + H2O ®
–CHOH–COOH + 2H+ + + 2e–
or more simply, there has been a gain of an oxygen atom.
The term
glycoside or glycosidic bond does not appear in the syllabus document or in CCHSC but
the latter is mentioned in the 2007 Report from the Marking Centre, so maybe it
does need explanation.
This
rambling account is meant to put a little more chemistry behind the test for
reducing and non-reducing sugars that is required for the HSC, though all this
chemistry is not required for the HSC.
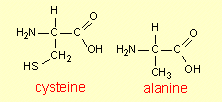
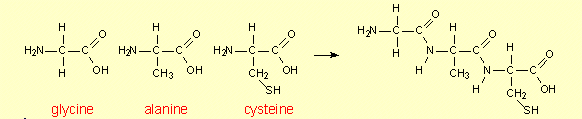
5. Peptide
formation
The formation of dipeptides and
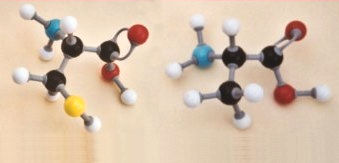
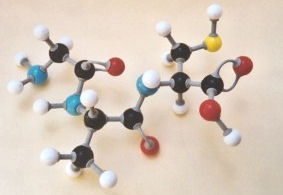
tripeptides is discussed in CCHSC on p 484-5 and molecular models of on
dipeptide is shown in Figure 14.2. It may be helpful to look at the
formation of another dipeptide and also of a tripeptide. The dipeptide
in Figure 14.2 is alanylcysteine. Now we shall consider the formation of
the other peptide that can be made from these two amino acids – by
using the other ends of the molecules to join them together.
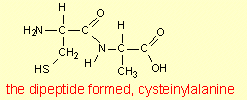
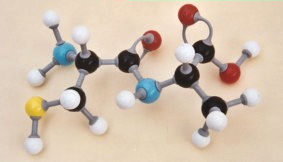
Formation of the dipeptide
cysteinylalanine
Formation of a tripeptide
using glycine, alanine and cysteine
6. DNA analysis If
you compare the account of DNA analysis in CCHSC with the accounts in other HSC
textbooks and study guides, you will find that CCHSC is the odd one out; the
other books describe processes involving restriction enzymes and radioactive
transfers from electrophoresis plates etc. Rest assured that the account in
CCHSC is the standard procedure currently being used in Australia for forensic
purposes, that is for identifying persons. The account in CCHSC was checked by a
senior scientist in a laboratory that routinely does DNA analysis for the NSW
police force and the results shown in Fig 14.16 came from a similar forensic
laboratory. The procedures described in the other texts date from the 1990s
before current methods were fully developed. It is a pity the HSC examiners in
2003 chose to use a very old electrophoresis plate for their exercise in
Question 34(d).
The earlier methods that cut up the DNA using restriction enzymes were not
nearly as sensitive as the current methods. It is the PCR amplification (p 502)
that makes current methods extremely sensitive (able to use very small
samples).
In addition those earlier methods were not conducive to setting up DNA data
banks, because they required that samples for comparison be run in parallel
using identical conditions for each and then comparing the electrophoresis
patterns as in the 2003 HSC exam question; these patterns were not just of a few
selected introns. The current method focuses on just ten introns by magnifying
(amplifying) them by a factor of over a million while leaving other introns
unaffected and therefore insignificant on the electrophoresis plate. It is
possible to prepare a mixture that contains all possible lengths of these ten
introns and this standard mixture can be run along side the test sample to allow the
lengths in the sample to be calculated (as in Fig 14.16 on p 504). Because
actual lengths of introns can be measured in this way a data bank can be
established. The data bank is dependent upon the introns being used. If a new
procedure was adopted that used a different set of introns, then the existing
data bank would become useless; DNA data banks are specific to the particular
set of primers (and introns) being used.
As explained on p 505 DNA analysis for forensic purposes is not the only type of
DNA analysis. For scientific and medical purposes restriction enzymes are widely
used to cut DNA molecules into smaller lengths which are then sequenced by
cutting off nucleotides one by one and identifying them by electrophoresis. In
such analyses radioactive labelling as an alternative to fluorescence tagging is
sometimes used. A variety of techniques of DNA analysis involving clever
interpretative skills is used for a range of medical and genetic studies; this
work is usually more detailed and complex than what has now become a fairly
routine method of DNA analysis for identification (forensic) purposes.
The common sources of DNA for forensic testing are saliva, blood, semen and hair
follicles (roots). Note that despite what you often see in TV crime shows hair
itself does not contain DNA: it's pure protein. You need the hair root to get
DNA. Exercise
| 1. |
Forensic DNA analyses for a family
consisting of a man, woman and three children are shown below.
What biological relationships exist between the children and the adults
in this family? (Answer at the bottom of the page)
| Location
on DNA molecule |
Number
of repeat units at the location |
| Woman |
Man |
Child
1 |
Child
2 |
Child
3 |
| A |
15,
18 |
12 |
13,
18 |
12,
19 |
12,
15 |
| B |
13,
19 |
20,
21 |
13,
20 |
14,
21 |
19,
20 |
| C |
22,
27 |
19,
27 |
22,
25 |
22,
27 |
27 |
| D |
9,
17 |
14,
16 |
17 |
14,
18 |
14,
17 |
| E |
30,
34 |
27,
34 |
26,
34 |
34,
37 |
27,
34 |
| F |
18 |
10,
11 |
11,
18 |
11 |
11,
18 |
| G |
8.
14 |
9,
15 |
8,
15 |
8,
15 |
8,
9 |
| H |
13,
15 |
11,
12 |
7,
15 |
9,
12 |
11,
13 |
| J |
7,
14 |
11,
15 |
7,
12 |
11,
14 |
14,
15 |
|
7. Functional groups
It
may be helpful to summarise the various functional groups that we have met
throughout the HSC course:
.
|
Functional group |
Name |
Endinga |
Example |
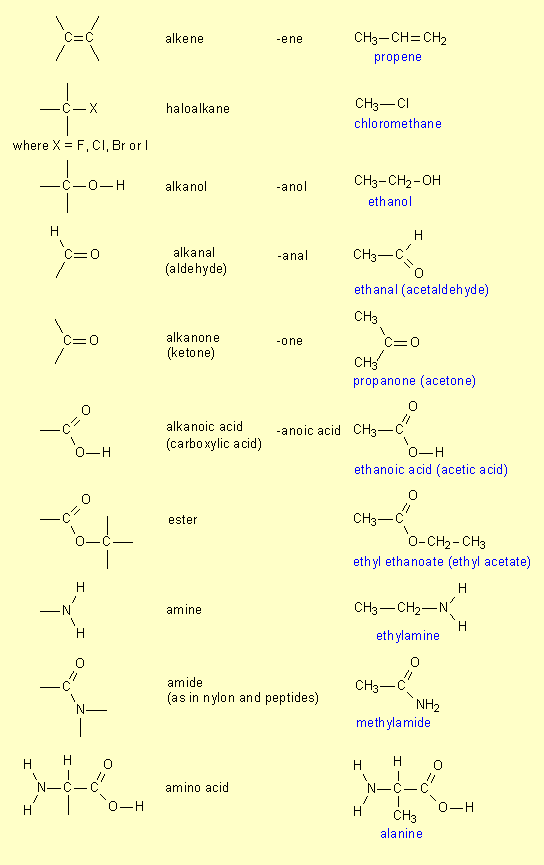 |
| a
Added to the stem, meth-, eth-, prop-, but- etc. |
8. Which analytical
technique for which classes of compounds? This option
considers several techniques for the analysis of very small samples – gas
chromatography, paper chromatography, HPLC, atomic emission and absorption
spectroscopies, electrophoresis, mass spectrometry. The question arises, which
technique is best for a particular class of compound. The following table show
which techniques are best suited to which class of compound (of those considered
in this option).
| Class
of compound |
Effective
analytical technique(s) |
| Carbohydrates |
HPLC |
|
Amino acids and short-chain (low molecular weight) polypeptides |
paper
chromatography, HPLC, electrophoresis |
|
Proteins |
electrophoresis (with or without hydrolysis) or (after
hydrolysis) paper chromatography or HPLC |
|
Contaminants in foods (such as pesticides) and illegal drugs (such as
opiates and steroids) |
Gas-liquid chromatography (particularly
capillary column GC) and HPLC, often with a mass spectrometer attached
for identification of individual compounds as they elute (leave the
column). |
Elemental composition of predominantly inorganic substances such as
soil, glass
and paint chips |
atomic emission spectroscopy |
|
Heavy metals in the environment |
atomic emission spectroscopy for
qualitative identification and approximate quantitative measurement and
atomic absorption spectrometry for accurate quantitative analysis of
specifically-targeted metals |
Gas chromatography requires the sample to be vaporised
for the analysis to occur. This means that it is not effective for classes of
compounds such as carbohydrates, amino acids and proteins because these classes
of compound generally decompose before they vaporise. Carbohydrates have very
high melting and boiling points because of the large amount of hydrogen bonding
between molecules which arises from the large number of OH groups in the
molecules. Amino acids are non-volatile because of their zwitterion (ionic)
structure. While this similar presence of charges on proteins is a factor in
their lack of volatility, the large molecular weight is also a major factor.
Carbohydrates, amino acids and proteins can be analysed by HPLC provided the
molecules are not so large that they have very low solubilities in available
solvents and stationary phases.
Exercises
(Answers at the bottom of the page)
| 2. |
Barbiturates are the most
widely used sedatives and relaxants, though they are addictive. They are
used in sleeping pills and in medications used to calm down over-excited
people. Unfortunately people can overdose on them and die, and
tragically some people use them for murder and suicide. Mixtures of
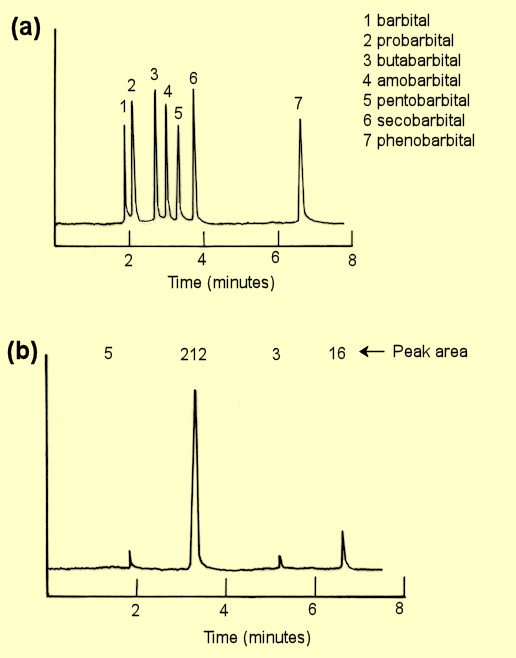
barbiturates can be analysed by gas-liquid chromatography. Figure (a)
below is a chromatogram of a mixture of seven known barbiturates. To try
to identify the drug that caused the death of a person, a forensic
chemist extracted any possible barbiturates from a sample of blood from
the deceased and performed a GLC analysis using conditions identical
with those used for chromatogram (a). The resulting chromatogram is
shown at (b). What is the main barbiturate in this sample? To help
investigators determine the source of the drug, identify any impurities
in the drug and determine the relative composition of the mixture. (It
may be helpful to print out these graphs.)
|
| 3. |
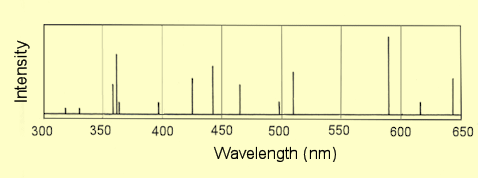
An analyst wished to
determine which of certain metal ions were present in the water from a
particular creek. The
metals of interest along with the wavelengths of their four or five
strongest emission lines are shown below. An emission spectrum of a
sample of the water was measured: it is shown below. Which of the metals
in the table are present in the water sample? Are there any emission
lines unaccounted for? Suggest some possibilities for these lines.
| Metal |
Wavelengths
of emission lines (in nm) |
| Ba |
389 |
455 |
554 |
649 |
|
| Cd |
361 |
442 |
509 |
644 |
|
| Cr |
358 |
361 |
425 |
465 |
|
| Cu |
282 |
325 |
522 |
578 |
|
| Fe |
358 |
372 |
382 |
386 |
|
| Hg |
365 |
405 |
436 |
546 |
579 |
|
Supplementary material (not
required for the HSC)
1. Diabetes and blood sugar
levels
Diabetes is a disease in
which the body is unable to regulate its blood sugar levels properly. If not
controlled, it can lead to fainting spells or comas, symptoms of malnutrition
(despite adequate food intake) and kidney and heart damage. It arises because
the pancreas is not producing sufficient of the hormone insulin.
Despite our intermittent eating habits, the human body is able to maintain
fairly uniform levels of blood sugar throughout the day. 'Blood sugar' is the
medical or everyday term for glucose, the predominant sugar carried by the
blood. Immediately after eating (particularly a meal rich in sugar), the glucose
concentration in the blood rises to quite high levels. This stimulates the
pancreas to secrete insulin which is needed to convert glucose to glycogen.
Glycogen is stored mainly in the liver. Glucose concentrations in the blood are
thus reduced. As body cells use up the blood glucose, glycogen is converted back
to glucose. In this way a fairly constant concentration of glucose in the blood
is maintained for several hours after a meal. If the meal had been low in
glucose but high in starch, then the body would slowly hydrolyse the starch and
supply a steady supply of glucose to the blood stream without the intervention
of insulin and glycogen.
If the pancreas is unable to make insulin (or to
make enough of it), glucose is not stored as glycogen in the liver.
Glucose concentrations in the blood remain high and the kidneys start
removing it and passing it to the urine. In
this way glucose is fairly quickly removed from the body.
This has two bad effects.
First the body becomes starved of glucose, since body cells quickly use
up what the kidneys have not removed. Secondly
high concentrations of glucose in the kidneys lead to greatly increased urine
output and cause considerable strain on the kidneys.
This in turn causes heart disease.
The brain gets all its energy from glucose and is the
first body organ to suffer if glucose levels drop off dramatically. This can
lead to collapse or coma.
Diabetes may be
a genetic disorder, but can also be the result of an infection in the pancreas
which permanently damages its ability to generate insulin. Diabetes in very
young children is generally the result of a viral infection. The genetic
disposition to the disease usually shows up in middle-aged and older people.
Diabetes can be exacerbated by obesity.
Mild cases of
diabetes can be controlled by diet. By
eliminating or greatly reducing the intake of mono- and disaccharides (which are
very quickly transferred to the blood as glucose), the sufferer prevents glucose
concentrations rising to high levels after eating.
The use of starch as the energy source results in a slow release of
glucose to the bloodstream over a period of several hours after eating. And
by having frequent small meals or snacks the lack of energy storage in the liver
is overcome.
Some cases of
diabetes can be controlled by drugs which stimulate the pancreas to produce
sufficient amounts of glucose. Severe
cases require daily injections of insulin.
Diabetics can
suffer two types of coma, one resulting
from too high a level of glucose, the other from too low a glucose level.
The first type is recognised by the sweet smell of acetone on the breath
and needs a shot of insulin to cure it. The
second type is caused by a very low glucose concentration
which starves the brain; this coma is overcome by administering glucose.
Diabetics often carry sweets (high glucose content) as a safety
precaution. Because these comas can easily
be mistaken for drunkenness, diabetics generally wear a bracelet or neck chain
identifying them as diabetics.
The existence and
control of this disease emphasises the complex interplay of energy intake,
storage and utilisation that goes on in the human body.
2.
Glycemic index, G.I.
An important aspect of food, particularly for
diabetics and people needing to watch their weight carefully is glycemic index.
The glycemic index G.I. of a food is a measure of the speed at
which glucose is released from that food by the human body to the blood stream.
A high G.I. means that glucose is released quickly; a low value means it is
released more slowly.
Essentially the G.I. of a food
is the amount of glucose released to the blood stream over a two-hour period
after eating a quantity of the food expressed as a percentage of the amount of glucose
released after eating a mass of pure glucose equal to the mass of carbohydrate
in the food being tested. So glucose has a G.I. of 100.
The table below classes some common foods in terms of their G.I. (low, medium or
high).
A food with a high glycemic index rapidly produces
glucose. This is a problem for diabetics as explained above but can also be a
problem for other people because the glucose is rapidly converted to glycogen
and as glucose levels fall the person begins to feel hungry again and may
overeat and become overweight. A food with a low glycemic index releases glucose
more slowly over a longer period of time and so the person does not feel hungry
quite so quickly and can more easily manage food intake.
It has been found that eating foods with low glycemic
indices can help people manage their diet and weight more easily.
It is perhaps surprising that foods with high starch
contents can have quite different glycemic indices. This is because the
starch can be present in different physical forms and mixed up with other
substances such as cellulose in different foods and this makes it harder for
body enzymes to get at it. Fructose, a major sugar in fruit, has a low G.I.
because, though a monosaccharide, fructose cannot be used directly as an energy
source by the body. Fructose first has to be converted to glucose and that takes
time which is one reason why fruit is a very good food.
| Low
G.I. foods (<55) |
Medium
G.I. foods (55 to 70) |
High
G.I. foods (>70) |
| pasta
(spaghetti) |
wholemeal
bread |
white
bread |
| grainy
breads |
basmati
rice |
potatoes |
| soya
beans, lentils |
weetbix,
vita-brits |
white
long-grain rice |
| carrots |
orange
juice |
cornflakes,
rice bubbles |
| apples,
pears, oranges |
bananas |
sweets
(jelly beans, lifesavers) |
| milk,
yoghurt, ice cream |
honey |
soft
drinks, sports drinks |
| muesli |
sucrose |
glucose |
| fructose |
|
|
3.
How fussy can we get? Galactosemia and phenylketonuria (PKU)
The two genetic disorders, galactosemia and phenylketonuria, illustrate
just how fussy the human system is. Very
slight differences in molecules can cause big problems.
Galactose is a monosaccharide which is very similar to glucose; the two
compounds differ only in the positioning of one OH group (CCHSC Figure
13.2 on p 466). Galactose and glucose are the monosaccharides that make up the
disaccharide lactose that is present in many foods, particularly in milk. The cells
of our body are not able to metabolise galactose directly. 'Normal' humans have an enzyme
that catalyses the conversion
of galactose to glucose. While the presence of galactose in most parts of the body causes no
problem, the brain is severely damaged by it. This leads to mental retardation.
Genetically some people do not have the necessary enzymes for the
conversion of galactose to glucose. They
suffer brain damage if the problem is not detected very early in life,
particularly as all milk – cow and goat as well as human – contains lactose
which hydrolyses to galactose and glucose.
People with this disorder called galactosemia need a special diet that excludes all foods
that contain galactose (mainly dairy products).
Phenylalanine and tyrosine are common amino acids. They differ in that tyrosine has an OH group on the benzene ring:
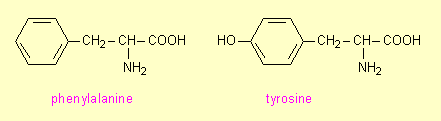
The human body does not use phenylalanine. Instead it has an
enzyme phenylalanine hydroxylase that converts it to
tyrosine which the body does use: it incorporates tyrosine into many of the
structural and functional proteins of the body. Genetically some people lack this enzyme. Hence phenylalanine builds up to quite significant concentrations in the
blood and this causes brain damage. The
condition is called phenylketonuria or PKU.
If it goes undetected, it causes severe mental retardation.
Fortunately simple blood tests are available for detecting both of these
conditions. In Australia all babies
are routinely tested a couple of days after birth for four conditions – the two
discussed here plus cystic fibrosis and hypothyroidism.
The combined test is called the Guthrie test or just the Blood Spot test.
Until a few years ago PKU was detected by testing the urine of young
babies.
The method of controlling PKU is to restrict very greatly the amount of
phenylalanine in the diet. The
widely used artificial sweetener, aspartame (trade name Nutrasweet) must be
avoided, because it hydrolyses to aspartic acid and phenylalanine in the
digestive tract. Controlled diet is
particularly important for babies and very young children.
When more mature, PKU sufferers are able to tolerate higher
concentrations of phenylalanine. This
means that diets can be considerably relaxed. However female PKU sufferers must revert to strict diets several months
before becoming pregnant, because levels of phenylalanine which are harmless to
the mother can cause irreparable brain damage to the unborn baby.
These two disorders illustrate just how precise the chemistry of living
systems is; very small changes in structures can have quite drastic
consequences.
4. Hair perms
Permanent waving of hair is a simple example of how the secondary and
tertiary structure of a protein can be modified. The main constituent of hair is the protein, keratin. It contains considerable amounts of the
sulfur-containing amino acid
cysteine (CCHSC Table 14.1 on p 482). This means
that sulfur-sulfur bridges (p 487)
play an important role in determining the secondary and tertiary structure of
keratin.
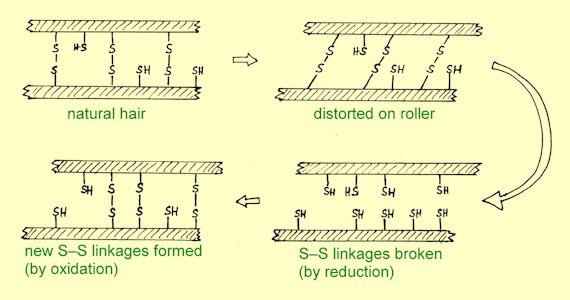
The 'curliness' or
'straightness' of hair fibres can
be altered by modifying these S–S bridges.
First the hair is treated with a reagent which breaks S–S links and
reduces them to SH groups as in the original cysteine.
The hair fibres are then distorted by wrapping around rollers, and
finally the hair is treated with another chemical which oxidises nearby SH
groups to new S–S bridges. Because
different SH groups become lined up when the hair is rolled, the resulting
overall structure is different from the starting one. The diagram illustrates the procedure.
This procedure is
'permanent' in that these new S–S links
remain intact, but of course as the hair grows, the new hair has the original
(genetic) alignment of the fibres. Hence
permanent waves eventually grow out.
Answers
to exercises
| 1. |
The woman and man are
the parents of Child 3. The woman is the mother of Child 1 but the
man is not the father. The man is the father of Child 2 but the
woman is not the mother. Most likely what we have here is a
so-called blended family; Child 1 is the offspring of a previous
relationship (marriage) of the woman with another man while Child
2 is from an earlier relationship (marriage) of the man with a
different woman – sometimes referred to as a his, mine and ours
family. |
| 2. |
The drug used in
pentobarbital. Impurities are probarbital and phenobarbital, along
with another compound that cannot be identified (retention time
5.2 minutes). Pentobarbital is approximately 90% of the mixture.
There is 2% probarbital, 7% phenobarbital and 1% unknown compound
(assuming that the sensitivity of the detector is the same for all
compounds in the analysis and that peak area is proportional to
amount of compound which would be only approximately true).
Because different scales are used in the two chromatograms you
have to measure the retention time for each peak. In (a) retention
times are:
peak 1, 1.9 m; 2, 2.1 m; 3, 2.7 m; 4, 3,0 m; 5, 3.3 m; 6, 3.7 m;
7, 6.6 m. In (b) main peak, 3.3 m; minor peaks, 1.9 m, 5.2 m, 6.6
m. |
| 3. |
The metal ions present
are Cd and Cr. Lines unaccounted for are 318, 330, 363, 397, 498,
590, 616. Some of these lines probably come from sodium which is
likely to be the most abundant cation in the water. Other cations
that could be present are calcium and magnesium and possibly
potassium. |
260213
|

 or
or