Further Exercises
If you are using a dial-up internet connection, I suggest that you work these exercises 'off-line' (to save Internet
connection charges and to free up the telephone). The answers are part of this file so both exercises and
answers are now temporarily in your computer. Go off line (File, Work
offline) and disconnect from your ISP (going off line in Explorer
does not automatically do this). Now you can work exercises and check answers
by clicking the relevant entries in the Contents frame.
Alternatively you can print out the exercises and answers (File, Print,
Only the selected frame).
| A1 |
Explain
the difference between catalytic cracking and steam (or thermal) cracking.
Give equations to illustrate. What is the main purpose of each process?
|
| A2 |
(a) |
Write
an equation using molecular formulae for the combustion of pentane. |
|
(b) |
Write
an equation for another common reaction that pentane undergoes. Name the
type of reaction.
|
| A3 |
When
propane reacts with chlorine in the presence of u.v. light, a great variety
of compounds is formed. |
|
(a) |
How
many monosubstituted compounds can be formed? Draw their structures. |
|
(b) |
Write
a balanced equation for the formation of one of these. |
|
(c) |
How
many disubstituted compounds can be formed? Draw their structures.
|
For pages 10-11
| B1 |
Write equations
using structural formulae for the reaction of 2-pentene with |
|
(a) |
hydrogen (with a
nickel catalyst) |
(d) |
hydrogen chloride |
|
(b) |
bromine in a
non-aqueous solvent |
(e) |
water (with
sulfuric acid catalyst) |
|
(c) |
bromine water
(bromine dissolved in water) |
|
|
|
Name
the compounds formed in (a), (d) and (e).
|
| B2 |
In
school laboratories to illustrate the test for distinguishing between
alkanes and alkenes teachers commonly use hexane and hexene. Why are
butane and butene not used? What is the test?
|
For pages 14 and 19
| C1 |
Write an equation
for the polymerisation of ethylene. What is the chemical difference between
low density and high density polyethylene? Briefly describe the industrial
processes for making these two products.
|
| C2 |
Which of the
following compounds would you expect to be able to form an addition
polymer?
CH2=CHBr, CHCl=CHCl, CH3–CH2Cl, CH3–CH2–CH2–CN,
CH3–CH=CHF,
HO–CH2–CH2–OH
For those that do, draw a segment of the polymer it would form.
|
| C3 |
For four common
addition polymers draw up a table showing a major use for each and the
property that makes it particularly suited for that use.
|
For pages 21-22
(There
are no exercises labelled D)
|
| E1 |
The
common nylon, called nylon-66, is made from
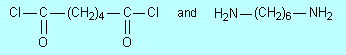
What small molecule is eliminated when these molecules polymerise? Draw a
segment of the polymer that forms; include at least two of each monomer
units.
Draw the structure of nylon-6 (page 20) under the structure of this nylon-66
and point out the similarities and differences between them.
|
| E2 |
Kevlar is a very
tough polymer; it is used for bullet-proof vests and sails for racing
yachts. It is made from H2N–C6H4–NH2
and HOOC–C6H4–COOH where C6H4
is a benzene ring (the same ring that is present in styrene and polyester
or PET). Draw the structure of a
segment of Kevlar. To which class of compound does it belong?
|
| E3 |
Silicones are
polymers containing Si, O, C and H. They are used as water-proofing
agents, breast implants, car polishes and synthetic rubber. The simplest
silicone is made from
(CH3)2Si(OH)2 (four
groups attached to a central Si atom). Draw the structural formula of this
compound. Is polymerisation of this compound addition or condensation
polymerisation? Draw a structure for a segment of the polymer to justify
your choice.
Polymerisation of (CH3)2Si(OH)2
leads to a linear-chain polymer. Show how use of some CH3–Si(OH)3
in the starting material would lead to chain branching. |
After studying Section
1.13
| E4 |
Give two (current
or future) problems associated with the use of present-day synthetic
polymers. Describe one recent development that has considerable potential
for overcoming these problems. Include any relevant chemical structure.
|
| E5 |
Explain how the
structure of cellulose is derived from that of glucose. Include some form
of chemical structure for cellulose. What is the 'stumbling block' in
using cellulose as a source of C2 to C4 chemicals
that we currently get from crude oil?
|
| E6 |
Name two products
in every-day use that are made from cellulose. Give a common use for each.
|
For page 28
| F1 |
(a) |
Draw the structure
of 2-pentanol. |
|
(b) |
Name the compound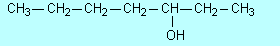
|
| F2 |
How
many compounds are there that could be named hexanol? Draw their
structures and name them.
|
| F3 |
The
compounds pentane and 1-pentanol have boiling points 138oC and
30oC. Which boiling point belongs to which compound? Explain
how you decided this.
|
| F4 |
If you
wanted to make a solution of each of the following substances, for which
ones would you use ethanol and for which water?
iodine, magnesium sulfate, perfume, a food colouring agent, sucrose.
|
For pages 32-33
| G1 |
Describe the
production of ethanol from plant material. Give sufficient detail to
justify it being a four-mark question in an HSC exam.
|
| G2 |
What mass of
ethanol do you need to burn to boil enough water to make a cup of coffee?
Assume that you need 250 g water of specific heat capacity 4.2 J
K–1 g–1, that it needs to be heated from 20oC
to 100oC, that the molar heat capacity of ethanol is 1360 kJ
mol–1, and that 50% of the heat is lost to the surroundings.
|
| G3 |
Describe how you
would experimentally determine the heat of combustion of 1-propanol.
Include a list of the actual measurements that you would make and explain
how you would calculate a value of the heat of combustion from them. Write
an equation for the combustion of 1-propanol. Identify two sources of
error in your experiment.
|
| G4 |
Discuss the use of
ethanol as a fuel for motor cars, including any environmental impacts of
such use. Assume that this is a five-mark HSC exam question.
|
For pages 39-40
| H1 |
(a) |
What
changes, if any, would you observe if |
|
|
(i) |
a piece of clean
shiny magnesium ribbon was dropped into a pale blue copper sulfate
solution |
|
|
(ii) |
a piece of silver
wire was dipped into another sample of the above copper sulfate solution |
|
|
(iii) |
a silvery granule
of zinc was dropped into a clear lead nitrate solution. |
|
(b) |
Write
oxidation and reduction half equations and the complete equation for each
of the reactions that occur in (a).
|
| H2 |
In the
following ionic compounds what is the oxidation state of |
|
(a) |
the
metal atom in (i) aluminium hydroxide (ii) lithium sulfate (iii)
titanium dioxide, TiO2 |
|
(b) |
the
non-metal atom in (i) iron(III) oxide (ii) sodium
fluoride
(iii) magnesium nitride, Mg3N2.
|
For page 45
| J1 |
A cell
consists of a silver wire dipping into a silver nitrate solution with a salt
bridge connecting this solution to a solution containing chlorine and sodium
chloride; a piece of platinum wire dips into this solution. Sketch what
this cell would look like.
Measurements with a voltmeter show that the platinum wire is positive with
respect to the silver wire. What electrode processes are occurring? Show
the direction of electron flow in the external circuit. What migration of
ions occurs (if any) as current flows? What is the overall cell reaction?
|
| J2 |
Sketch
a possible experimental arrangement for a galvanic cell which would
correspond to each of the following: |
|
(a) |
a Mg, Mg2+
electrode connected to a Ni, Ni2+ electrode |
|
(b) |
a hydrogen
electrode (page 57) connected to a Cu, Cu2+ electrode |
|
In
these cells the positive electrodes are (a) the nickel wire (b) the copper
wire. On each of your sketches indicate the reaction that occurs at each
electrode when the electrodes are joined with a conducting wire. Show the
direction of flow of electrons in the wire and show the direction of
any ion migration that occurs in the cell. For each cell write the overall reaction.
|
| J3 |
For
each of the cells in exercises J1 and J2 which electrode is the anode and
which the cathode?
|
For page 49
| K1 |
(a) |
Write the anode and
cathode reactions for (i) the Leclanché dry cell and (ii)
the alkaline cell. |
|
(b) |
What are the
oxidation states of the oxidised and reduced forms of the metals in these
reactions? |
|
(c) |
List three
similarities and three differences between these two cells.
|
| K2 |
Repeat
exercise K1
with the silver oxide cell instead of the alkaline cell.
|
| K3 |
(a) |
One type of lithium battery used in cameras
consists of a
Li, Li+ (in non-aqueous solution) electrode connected with a
potassium hydroxide paste to an electrode consisting of MnO2
and Mn(OH)3 in contact with an inert conductor. Write equations for the half reactions that occur at the electrodes (the
Li electrode is negative) and hence the overall reaction. |
|
(b) |
Another type of
lithium cell is used in heart pacemakers (implanted in the body). It is
completely solid state and lasts about ten years. In this cell one
electrode is lithium metal while the other is iodine dispersed in a
conducting polymer; these electrodes are separated by crystalline lithium
iodide. Again the lithium electrode is negative. Deduce the electrode reactions
and hence the overall reaction. |
|
(c) |
Why is it necessary
to exclude water from these cells?
|
After studying Section 2.13
| L1 |
Zinc reacts with
silver nitrate solution to form metallic silver. Would it be possible to
use this reaction to make a galvanic cell? If so explain how you would
make such a cell. Include a diagram. Give the electrode reactions and the
overall reaction and on your diagram show the flow of electrons in the
external circuit.
Would this cell be rechargeable? Why or why not?
|
| L2 |
As the lead
accumulator is discharged, the concentration of sulfuric acid decreases.
Explain why. On the other hand, as the nickel-cadmium cell is discharged
the concentration of hydroxide remains constant. Explain why. What, if
any, effect do you expect the decreases or constancy of electrolyte
concentration to have on cell voltage?
|
| L3 |
Why is the silver
oxide cell not rechargeable?
|
For pages 63-4
| M1 |
Explain
how you would measure the standard electrode potential of the Pb, Pb2+
electrode if you did not have a standard hydrogen electrode available (but
did have some other metal, metal ion electrodes available).
|
| M2 |
(a) |
Calculate the
standard EMF of the following reactions |
|
|
(i)
Cu2+ + Cd(s) ® Cd2+
+ Cu(s) |
|
|
(ii) 2Fe3+
+ 2Br– ®
Br2 + 2Fe2+ |
|
(b) |
What do your
answers tell you about the directions in which these reactions go? |
|
(c) |
Physically, what
does the 'EMF of a reaction' mean?
|
| M3 |
(a) |
A cell
consisted of a piece of copper wire dipping into a 1.00 mol/L copper
nitrate solution which was connected by a salt bridge to a 1.00 mol/L
chromium(III) sulfate solution into which dipped a piece of chromium. The
cell had an EMF (voltage) of 1.08 V, the copper wire being positive.
Knowing that the standard electrode potential of the Cu2+, Cu
electrode is +0.34 V, calculate Eo for the chromium
electrode. |
|
(b) |
A cell
consisted of a piece of platinum dipping into a solution containing both
iodine and iodide at unit molarity connected by a salt bridge to a 1.00 mol/L
solution of zinc sulfate into which dipped a zinc rod. The EMF of the cell
was 1.30 V (platinum wire positive). Knowing that Eo for
the zinc electrode is –0.76 V, calculate the electrode potential of the
iodine, iodide electrode. |
| M4 |
Two
galvanic cells were made as follows: |
|
|
(i) |
A piece of platinum
wire dipped into a solution that was 1.00 mol/L in each Fe2+
and Fe3+. A salt bridge connected this solution to another one
which was 1.00 mol/L in lead nitrate and which had a strip of lead metal
dipping into it. |
|
|
(ii) |
A piece of silver
wire dipped into a 1.00 mol/L silver nitrate solution which was connected
by a salt bridge to another solution which was 1.00 mol/L in iron(II)
sulfate; a strip of iron metal dipped into this. |
|
(a) |
Calculate
the standard EMFs of these cells. |
|
(b) |
Write
the chemical reactions that occur in these cells as they generate
electricity. |
|
(c) |
Draw a
diagram for each cell. Include some form of external circuit (for example,
light bulb or voltmeter) On your diagrams show the way electrons and ions
flow through the cells and external circuits.
|
For pages 72-3
| N1 |
How
many protons, neutrons and electrons are there in the atom and ions
represented by the following symbols (X, Y and Z are not chemical
symbols): |
|
(a) 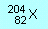 |
(b) 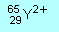 |
(c) 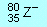
|
| N2 |
Complete
the following nuclear equations: |
|
(a) |
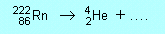 |
|
(b) |
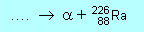 |
|
(c) |
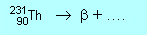 |
|
(d) |
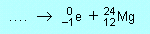 |
| N3 |
Use the Periodic
Table to decide which element is formed when each of the following
undergoes alpha decay
(a) thorium-234 (b) uranium-235 (c) lead-210?
What element is formed when the following elements undergo beta decay:
(d) thorium-234 (e) sodium-24 (f) iodine-131?
|
| N4 |
Which of the
following elements would you expect to be unstable? Why?
(a) 232Th (b) 20Ne (c) 40Ca
(d) 27Mg (e) 3H
|
For page 76
| P1 |
If plutonium-239 is
bombarded with an alpha particle of sufficient energy for the two
particles to stick together to form an atom, what element would it be?
Write a nuclear equation for the reaction.
|
| P2 |
One product from
fission of uranium-235 is caesium-137. Write a possible nuclear equation
for the formation of caesium-137. (More than one equation is possible,
depending upon whether 1, 2 or 3 neutrons are produced.)
|
For page 85
| Q1 |
Iodine-131
has a half-life of 8 days. What percentage of a sample of this isotope
will be present after 40 days?
|
| Q2 |
Sodium-24
is a radioactive isotope. The radioactivity of a particular sample of
sodium-24 was measured in counts
(emissions) per second at various times from the start of an experiment
(taken as time zero). Results are given in the following table
| Time
(hours) |
0 |
5 |
10 |
15 |
20 |
25 |
30 |
| Counts
per second |
464 |
370 |
292 |
232 |
185 |
146 |
116 |
|
|
(a) |
What is the
half-life of sodium-24? Explain how you decided this. |
|
(b) |
How long after the
start of the experiment will it be until the disintegration rate for this
sample is 58
counts per second? |
|
(c) |
Sodium-24 decays by
beta emission. Write an equation for this decay process.
|
| Q3 |
Describe
(in sufficient detail to justify four marks in an HSC exam) one practical
application (medical or industrial) of the use of a
radioisotope. Include an explanation of how the method works and of how the
results are interpreted.
|
Answers to Further Exercises
E1 |
HCl;
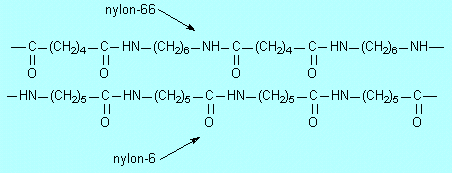
In these structures –(CH2)5– means –CH2–CH2–CH2–CH2–CH2–
and similarly with the 4 and 6.
Similarities: (1) both use the same amide
(or peptide) –CO–NH– linkage
(2) both have six C atoms between the NH groups (3) both are linear chains
(no branching)
Differences: (1) In nylon-6 the repeat C6 units are all identical, –(CH2)5–CO–
whereas in nylon-66 alternate ones are different, –CO–(CH2)4–CO–
and –(CH2)6– (2) the CO groups are always 6
atoms apart along the chain in nylon-6 while in nylon-66 they alternate
between 4 and 8 atoms apart. |
| E2 |
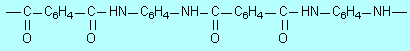
It is a nylon or polyamide or just a
condensation polymer. |
| E3 |
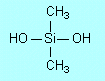
Condensation, because molecules of water are
eliminated between pairs of OH groups (on adjacent (CH3)2Si(OH)2
molecules) to form
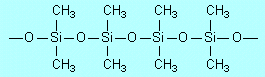
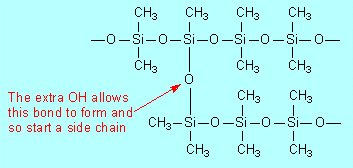
|
| E4 |
Depletion
of raw materials (crude oil) (pages 18-19), lack of biodegradability (page
24).
Biological synthesis of poly(b-hydroxyalkanoates)
such as
poly(b-hydroxybutanoate)
– describe it with a structure (see pages 24-5) |
| E5 |
By
glucose molecules joining together by elimination of molecules of water
(see middle of page 20). The simplest structure for cellulose would be
–O–C6H10O4–O–C6H10O4–O–C6H10O4–O–C6H10O4
Alternatively you could use one of the following:
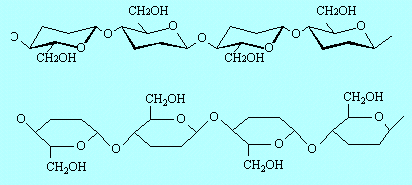
(There are two OH groups and four H atoms attached to each ring that are
not shown in these diagrams.)
The 'stumbling block' is breaking the cellulose into glucose. Glucose is
easily converted to ethanol by fermentation (see page 23). |
| E6 |
Any two
of rayon (textiles), cellophane (wrapping), cellulose acetate (overhead
projector slides), cellulose nitrate (explosive), carboxymethyl cellulose
(food thickener).
|
| F1 |
(a) |
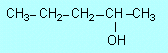 |
|
(b) |
3-heptanol |
| F2 |
3;
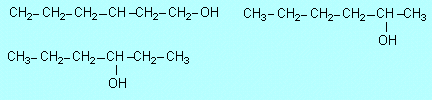
1-hexanol, 2-hexanol, 3-hexanol |
| F3 |
pentane,
30oC; 1-pentanol, 138oC. Hydrogen bonding occurs in
1-pentanol so it has strong intermolecular forces and so has the higher
boiling point. Pentane has only dispersion forces. |
| F4 |
ethanol:
iodine, perfume, a food colouring. Could use either for sucrose.
|
| G1 |
Describe
fermentation and make sure you include at least four significant
facts: an equation, mention yeast, conditions to use (blood temperature,
exclusion of air), maximum concentration produced, distillation (see pages
28-30). |
| G2 |
5.7 g |
| G3 |
Burn
1-propanol in a small spirit burner and use the heat to warm up some
water in a container held over the flame. See CCPC page 291-2.
Measure the mass of burner plus propanol before and after the burning.
Measure the mass of water used and measure its temperature before and
after heating. See Box 10.1 on page 292 for the calculation.
C3H7OH + 5O2 ®
3CO2 + 4H2O |
| G4 |
See
pages 30-31. You need at least five significant facts. Ethanol can be used
as an additive to normal petrol (to about 20%) without any modification to
the car engine, or with engine modification 95% ethanol can be used as a
total replacement for ordinary petrol; this was tried in Brazil in the
1970s and 80s but was abandoned because of cost factors. You could include
the equation for combustion of ethanol.
The main reason for wanting to use ethanol is to reduce the present rapid
rate of consumption of oil which is likely to run out within a few
decades. The other reason is its possible reduced contribution to the
enhanced greenhouse effect. Currently ethanol is more expensive than
petrol even when it is made from wastes such as molasses from sugar cane
and it is dearer still if crops are grown specifically for making ethanol
Key environmental issues are contribution to easing (or aggravating) the
greenhouse effect (depending on where the energy for the distillation
comes from), disposal of wastes from the fermentation process and possible
land degradation resulting from putting large areas of land under crops.
|
| H1 |
(a) |
(i) |
he magnesium gets
covered with brown copper while the blue copper sulfate solution becomes
paler |
|
|
(ii) |
no
change |
|
|
(iii) |
The
zinc becomes covered with a black deposit of lead metal. |
|
(b) |
(i) |
Mg ®
Mg2+ + 2e–; Cu2+ + 2e– ®
Cu
Mg(s) + Cu2+(aq) ®
Mg2+(aq) + Cu(s) |
|
|
(ii) |
no reaction (see
relative positions of Cu and Ag in the Activity Series on page 36.) |
|
|
(iii) |
Zn ®
Zn2+ + 2e–; Pb2+
+ 2e– ®
Pb
Zn(s) + Pb2+(aq) ®
Zn2+(aq) + Pb(s) |
| H2 |
(a) |
(i)
+3 (ii) +1 (iii) +4 |
|
(b) |
(i)
–2 (ii) –1 (iii) –3
|
| J1 |
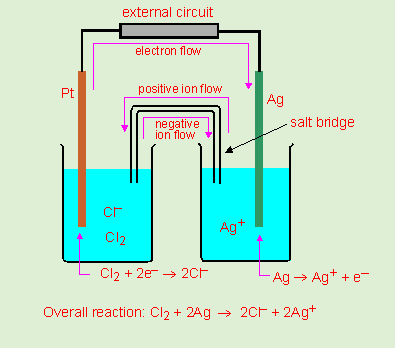
|
| J2 |
(a) |
Diagram similar to
the one in answer J1 with Mg and Mg2+ replacing Ag and Ag+
and with Ni replacing Pt and Ni2+ replacing Cl2, Cl–.
Electron and ion flow is as in J1. Electrode reactions are
Mg ®
Mg2+ + 2e– and Ni2+ + 2e– ®
Ni
Overall reaction: Mg(s) + Ni2+(aq) ®
Mg2+(aq) + Ni(s) |
|
(b) |
Diagram as in the
left-hand one of Fig 2.12 on CCHSC page 57 with Cu and Cu2+
replacing Zn and Zn2+. In the external circuit electrons flow
from the Pt wire to the copper. Positive ions flow from the left-hand
beaker to the right-hand one while negative ions flow from right to left. |
| J3 |
Anode:
in J1 the silver electrode; in J2(a) the magnesium electode; in J2(b) the
hydrogen electrode
|
| K1 |
(a) |
(i) Zn +
2MnO2 + 2NH4+ + 2H2O ®
Zn2+ + 2Mn(OH)3 + 2NH3
(ii) Zn + 2MnO2 + 3H2O ®
ZnO + 2Mn(OH)3 |
|
(b) |
In both cells: +2
and ) for zinc, +4 and +3 for Mn |
|
(c) |
Similarities: (i)
The same metals undergo the same changes in oxidation state (or
essentially the same reaction in both cells) (ii) generally made to look
similar and to be interchangeable (iii) both are non-rechargeable.
Differences: Any three of (i) The electrolyte paste in the Leclanché cell
is slightly acidic (NH4Cl) whereas in the alkaline cell it is
highly basic (alkaline) (ii) the alkaline cell can deliver much higher
currents (iii) The alkaline cell can deliver more total electricity from a
cell of the same size (iv) Alkaline cells are more expensive (v) Alkaline
cells can cause more damage if they leak. |
| K2 |
(a) |
(i) Zn +
2MnO2 + 2NH4+ + 2H2O ®
Zn2+ + 2Mn(OH)3 + 2NH3
(ii) Zn + Ag2O ®
ZnO + 2Ag |
|
(b) |
In the Leclanché
cell:+2 and 0 for Zn and +4 and +3 for Mn
In the silver oxide cell: +2 and 0 for Zn and +1 and 0 for Ag. |
|
(c) |
Similarities: (i)
Basically the same anode reaction (oxidation of Zn) (ii) both are
non-rechargeable (iii) Neither is able to deliver high currents.
Differences: Any three of (i) They have very different cathode reactions
– reduction of Mn in one, reduction of silver in the other (ii) Silver
oxide cells are more easily made very small (iii) they can deliver more
total electricity for a given size (iv) are more expensive and (v) have a
longer shelf life. |
| K3 |
(a) |
Li ®
Li+ + e–; MnO2 + 2H2O + e–
®
Mn(OH)3 + OH–
Overall: Li + MnO2 + 2H2O ®
Li+ + Mn(OH)3 + OH– |
|
(b) |
Li ®
Li+ + e–; I2 + 2e– ®
2I–
Overall: 2Li + I2 ®
2Li+ + 2I– |
|
(c) |
Because Li reacts
with water (forming H2 and LiOH)
|
| L1 |
Yes;
set up a cell similar to the right-hand one of Figure 2.4 on page 44 of
CCHSC with a silver wire and silver nitrate solution replacing the copper
rod and copper sulfate solution.
Zn ®
Zn2+ + 2e–; Ag+ + e– ®
Ag;
Zn(s) + 2Ag+(aq) ®
Zn2+(aq) + 2Ag(s)
Electrons would flow out of the zinc rod through the external circuit and
into the silver wire.
No, because as you tried to recharge it (by connecting a voltage source to
the cell with the voltage source's negative terminal connected to the
zinc) hydrogen ions or water would be reduced to H2 in
preference to Zn2+ being reduced back to Zn (note the relative
positions of Zn and H in the Activity Series). |
| L2 |
Because
the overall cell reaction uses it up; adding the two electrode reactions
from near the bottom of page 55 gives the overall reaction
Pb(s) + PbO2(s) + 4H+(aq) + 2SO42–(aq)
®
2PbSO4(s) + 2H2O(l)
Because the overall cell reaction does not consume hydroxide; adding the
two electrode reactions near the bottom of page 54 gives the overall
reaction
Cd(s) + NiO2(s) + 2H2O(l) ®
Cd(OH)2(s) + Ni(OH)2(s)
In the nickel-cadmium cell, because the electrolyte concentration remains
constant, the cell voltage remains constant (until one of the reactants is
nearly all consumed then it falls dramatically to zero). In the lead
accumulator as the concentration of sulfuric acid decreases the cell
voltage drops, because cell voltage depends upon concentration of the
species involved in the cell reactions; as the concentrations of reactants
decrease or of products increase the voltage drops. |
| L3 |
Because
the anode reaction,
Zn ®
Zn2+ + 2e– (or its alkaline equivalent, Zn + 2OH–
®
ZnO + H2O)
is not easily reversed by applying a voltage in the
opposite direction: water is reduced to H2 instead.
|
| M1 |
Set up
a cell using the Pb, Pb2+ electrode and a metal, metal ion
electrode of which the standard electrode potential was known and measure
its EMF. For example using a copper electrode (eo
= +0.34 V) set up the cell
Pb | Pb2+ || Cu2+ | Cu
and measure its voltage (0.47 V, Cu positive), then using Equation 2.12
from page 62
0.47 = 0.34 – eoPb
so eoPb
= –0.13 V |
| M2 |
(a) |
(i) +0.74 V
(ii) –0.32 V |
|
(b) |
(i) goes as written
while (ii) goes in the reverse direction (Br2 oxidises Fe2+) |
|
(c) |
It is the EMF
(voltage) of the galvanic cell in which the cell reaction is the given
reaction: in the case of (i) it is the voltage of the cell
Cd | Cd2+ || Cu2+ | Cu |
| M3 |
(a) |
–0.74 V |
|
(b) |
+0.54 V |
| M4 |
(a) |
(i) 0.90 V (Pt
positive) (ii) 1.25 V (Ag positive) |
|
(b) |
(i) Pb ®
Pb2+ + 2e–; Fe3+ + e– ®
Fe2+
(ii) Fe ®
Fe2+ + 2e–; Ag+ + e– ®
Ag |
|
(c) |
(i) Diagram should
look like the one in Answer 13 on page 533 with Fe2+, Fe3+
replacing I2, I– and with Pb, Pb2+
replacing Zn, Zn2+.
(ii) Again like Answer 13 on page 533 with the right hand beaker having a
silver wire and an Ag+ solution and the left hand one Fe metal
and Fe2+ in solution. |
| N1 |
(a)
82 p, 122 n, 82 e
(b) 29 p, 36 n, 27 e
(c) 35 p, 45 n, 36 e |
| N2 |
 |
| N3 |
(a)
Ra (b) Th (c) Hg
(d) Pa (e) Mg (f) Xe |
| N4 |
(a)
because its atomic number is greater than 83;
(d) because its neutron to proton ratio is outside the zone of stability:
it is in the blue region of Figure 3.1 on page 69, whereas the stable zone
is the grey region;
(e) same reason as (d)
|
| P1 |
curium,
Cm
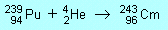 |
| P2 |
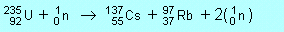
Alternatively you could have Rb-98 + 1n or
Rb-96 + 3n
|
| Q1 |
3.1% |
| Q2 |
(a) |
15 h; this is the
time required for the number of counts per second to drop to half – from
464 to 232, or from 370 to 185 or from 292 to 146 etc |
|
(b) |
45 h |
|
(c) |
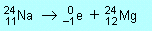 |
| Q3 |
Possible
applications include
(a) medical) (i) use of cobalt-60 for cancer treatment (ii) use of
technetium-99m for medical diagnosis
(b) industrial) (i) thickness gauges (ii) smoke detectors (iii)
irradiation of medical supplies and/or food
These are ones that are described in CCHSC pages 81-4. There are
numerous others.
For a four-mark HSC question you need to include at least four significant
facts. Before deciding which application to describe mentally go over what
you know about the method and make sure you have at least four key facts
to include: otherwise consider do a different application. On all of the applications listed above there is enough information in CCHSC
to score full marks. If short on facts, you could pad out your answer by including the
nuclear equation for the disintegration involved or by mentioning some
safety precautions, but make sure your 'story' holds together and is not
just a collection of disjointed facts. |
|