Errors
in Conquering Chemistry 4th edition HSC Course
To
return to where you came from click on the BACK
button in your web browser
If
you discover any other errors, please let me know on rolandh@tpg.com.au.
| Page
24, Exercise 28 b |
ii
should be 3-chloro-1-propanol (not 1-chloropropanol)
iii should be 1,3-dichloropropane (not
1,3-dichloropropanol) |
| Page
26, Figure 1.8 |
see
Section 1.18 (not 1.16) |
| Page
52, 3rd equation |
should be
Cl2(g) (not Cl2(aq) |
| Page
70 |
In the
line after the sub-heading A consequence '..that last
calculation ...' should be '... that last statement ...' |
| Page
120, Exercise 12 |
In the
Solubility row of the table 0.0165 (not 0.165) |
| Page
123 |
3rd line
above the sub-heading Environmental effects, (PANs not
PANS) |
| Page
136 |
2nd line
above the heading 4.18 Neutral ..., 'In Section
4.2...' (not 4.1) |
| Page
137, Table 4.6 |
on the
right, ACIDIC (not ACID) |
| Page
139, Table 4.7 |
pH range
for litmus 6.0–8.0 (not 5.0) |
| Page
143, Exercise 36 |
1st line,
1.8 (not 1.82) |
| Page
147 |
caption
to top photo, 'Antoine Lavoisier ...' (not Anotinie) |
| Page
155 |
5th
paragraph (ignoring equations), 5.2 should be 5.4 (twice) |
| Page
176, Table 5.9 |
In the
row for 'orange', octyl ethanoate (e missing) |
| Page
177, Exercise 34 |
In c
the structure should be 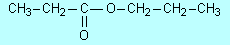
(=O wrongly placed) |
| Page
178, Exercise 39 |
Table
5.8 (not 5.9) |
| Page
214 |
3rd line
below the sub-heading Other tests for phosphate, ' ... of
our other anions ...' (insert other) |
| Page
218, Exercise 26 |
In 2nd
line of a change 'green' to 'brown' |
| Page
249,
Figure 7.4 |
The
bottom right structure is 1,1,3-trichloro-1,3,3-trifluoropropane |
| Page 250,
middle of the page |
Rule 5
should read ' ...gives the lowest number(s) to the halogen(s) in
alphabetical order: for example 1-bromo-2-fluoroethane not
2-bromo-1-fluoroethane and
1,1,3-trichloro-1,3,3-trifluoropropane not
1,3,3-trichloro-1,1,3-trifluoropropane'.
That is, delete ' ...to the most electronegative halogen, the
order of electronegativity being F > Cl > Br > I',
insert the bromofluoro example and change the numbering in the
propane example. |
| Page 250,
paragraph after Isomers heading |
5th line.
Change '1,3,3-trichloro-1,1,3-trifluoropropane' to
'1,1,3-trichloro-1,3,3-trifluoropropane'. (The name under the
right hand structure below this is correct.) |
| Page 251,
Table 7.5 |
Third
row. Change '1,2,2-trichloro-1,1,2-trifluoroethane' to
'1,1,2-trichloro-1,2,2-trifluoroethane'. |
| Page
251, Figure 7.5 |
In the
caption 'yellow is Cl ...' (not S)
Under the right hand structure the name should be
'1,1,2-trichloro-1,2,2-trifluoroethane' (not
1,2,2-trichloro-1,1,2-trifluoroethane) |
|
|
|
|
| Page
252, Question 13 |
d
should be 1,2-dichloro-1,1,2,2-tetrafluoroethane
*e should be ...2,2,3-trifluoropropane (not
...-1,2,3-trifluoro...)
(The answer for d on page 564 is correct.) |
| Page
301, Question 14 |
Should be
'phosphorus' (not phosphorous) |
| Page
319, Equation 9.7 |
Insert 2
in front of NH3 |
| Page
322, Exercise 6 |
In a
it should be Ag+(aq) (A missing) |
| Page
327, Exercise 15 |
The arrow
in the equation should be the normal reverse arrow 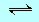 |
| Page
330, Table 9.3 |
In c
the values in the K column should be
3.6 x 104
2.2 x 103
(not 450, 30, 1.6, 0.21)
1.2 x 102
16 |
| Page
349 |
1st line
after Anode and cathode again, 'oxidation' (not
oxidatioon' |
| Page
350 |
1st line
after the sub-heading Electrolysis of dilute aqueous ...,
'sodium chloride' (not hydroxide)
6th line from bottom, '... the electrolysis of water.'
(not hydrolysis) |
| Page
352 |
second
dot point near bottom of page: should be '... forms unwanted
hypochlorite ...' (not chlorite) |
| Page
360, Exercise 12 |
Top left
box of the flow chart should be 1-butyl hexanoate (insert
1-) |
| Page
367 |
fourth
line after heading Anionic surfactants: formula should be
R–SO2–O– (delete extra O). Similarly
near the right hand end of the structure in the sixth line
delete the O between the benzene ring and the SO2 (SO2
should be directly attached to the benzene ring). |
| Page
388 |
Compulsory
experiments ... table: in Experiment 1, right hand column,
Chapter 9 Exercise 5 (not 4) |
| Page
410 |
2nd
bottom line, 'At the cathode (negative electrode)' should be
blue and bold type |
| Page
413, Exercise 25 |
3rd last
line, 'amount' should be 'rate' |
| Page
437 |
6th line
after the sub-heading Restoration of iron objects, 12.15
should be 12.16 |
| Page
474 |
1st line
after the heading 13.10 Ring and open-chain ..., Fig.
13.1 should be Fig 13.2 |
| Page
475 |
6th line
above the heading 13.11 Reducing and ..., a-glucose
should be b-glucose
5th line above that heading, b-glucose
should be a-glucose |
| Page
495, Exercise 20 |
2nd line
: ... travel down the paper ... (not up) |
| Page
529 |
Caption
to photo, bracket ) missing after 'to excite the atoms' |
| Page
544, Question 14 |
B is
2-hexene (not 1-hexene) |
| Page
544, Answer 20 |
–CH3
should be –H, and 'polypropylene' should be
'polyethylene" |
| Page
547 Exercise 25 a |
In iii
–2.24 not –2.50 |
| Page
553 Exercise 24 b |
0.74
L (not 11.9 L) |
| Page
561 Answer 1 |
The graph
is incorrect; it should be:
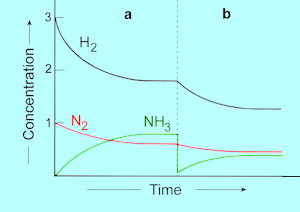
The sentence under the graph should read: At equilibrium ... 0.8
mol NH3 ...
and 1.8 mol H2 (not 0.4
and 2.8)
|
| Page
562 Answer 26 a |
FeCl3
(not FeCl2) |
| Page
563, Answer 12 f |
1,1,2-tribromo-1,2,2-trifluoroethane
(not 1,2,2- ... -1,1,2- ...) |
Page
564, Answer 15 d
Answer 17 |
The name
should be 1,1-dichloro-1,2,2,2-tetrafluoroethane
the name should be 1,1,1-trichloro-2,2,2-trifluoroethane |
Page
564,
missing Answer 16 |
a
trichloromethane
b dichloromethane
c 1-bromo-1-chloro-2,2,2-trifluoroethane |
| Page
587, Answer 3 b |
At the
end of the equation, '= 2Na+' should be '+ 2Na+' |
| Page
589, Answer 20 |
X
contains leucine, glycine and lysine
Y contains alanine, lysine and aspartic acid |
To
return to where you came from click on the BACK
button in your web browser
05022014
|