The views expressed in this Answers and Comments document are those of the author who had nothing to do with setting or marking the paper. The answers are not in any way 'official'. They are simply the responses of a competent chemist familiar with the syllabus and the textbooks in common use. The answers are probably a bit more detailed than would be required to gain full marks from HSC examiners.
The official examiners' comments and a marking scheme (of sorts) are available on the Board of Studies web site, www.boardofstudies.nsw.edu.au/hsc_exams/hsc2001exams/index.html (Click on it to go there; find Chemistry in the alphabetical list, click on the 'yes' in the exam report column (right column): after the file loads you could print it out in your normal way, but it is 41 pages! Alternatively you could save it to your hard disk and just view it on screen whenever you wanted to. To save it to your hard disc, right click the 'yes' then normal (left) click 'Save target as' and follow instructions. You need Acrobat Reader for this file to open; if you do not have this, you can find and download it by browsing around the Board of Studies web site – its free!))
The two major differences between this paper based on the new syllabus and previous papers based on the old syllabus are
| · |
There is a greater emphasis on straight recall of information and far fewer marks for problem-solving (though the balance is not too different from that in the 1999 specimen paper) |
| · |
There are questions that carry a large number of marks (5 to 7) but which do not give specific instructions about what is required. These are the hardest questions on the paper because you have to work out what the question really wants. Fortunately the questions of this type in the 2001 paper come from the 'Students do' column of the syllabus. |
1. Problem-solving versus bookwork
By my count there are 11 marks for numerical calculations, 16 ± 2 for qualitative problem solving and 73 ± 2 for straight recall of memorised material (the ± 2 arises because of the different options: two options have zero marks for problem solving, two have 2 marks and one has 4). To my mind this represents a very retrograde step: chemistry should be more about understanding basic principles and applying them to diverse situations rather than about assimilating vast amounts of factual material (that's going back to the 1950s!). I have no qualms about de-emphasising numerical calculations in favour of qualitative problem-solving but to shift the emphasis to recall of memorised information worries me greatly, particularly when it has been done without any specific policy statement to that effect (though in fairness the move could be inferred from the 1999 sample exam paper).
2. Extended response questions
In tackling extended response questions (questions that are just one or two sentences but worth 5 to 7 marks) you should start from the rule of thumb that the question requires one significant piece of information per mark, so a six mark question will require six pieces of relevant information. And it is going to require about 1.8 minutes per mark to answer as fully as the examiners want (11 minutes for a 6 marker). Analyse the question carefully before you start writing to make sure you understand exactly what the question is asking for. If it says 'describe' something and 'discuss' its importance or role in ..., then you need to split the marks between the two parts, say 3 for 'describe' and 3 for 'discuss'. If you feel that it is difficult to give 3 significant facts for the 'discuss' part, perhaps you could do a 4/2 split but a 5/1or 6/0 split would not be answering the question as asked. Even if you could easily give 6 significant facts for the 'describe' part, you must give some for the 'discuss' part even though that might be a struggle. Jot down a key word or two for each point you want to make. Only then are you ready to write your answer in the answer booklet. Note that point form presentation is acceptable (20001 HSC Notes from the Examination Centre Chemistry page 5).
Questions from the 2001 exam paper that fit into this extended response category are Questions 19, 22 and 25 from the core and parts (c) and (e) in each of the option questions. Although Questions 17, 18 and 24 each carry 6 marks, they are broken into parts which ask for specific information and for which the numbers of marks are given.
More comments about answering extended response questions will be given below when Questions 19, 22 and 25 are answered.
3. Key verbs
The Board of Studies has prepared a list of verbs that will regularly be used in exam papers and has defined their meanings fairly carefully. It is important that you know the meanings of these verbs and are able to distinguish between them. The ones that occur in the 2001 exam and which need care are: explain, evaluate, justify, compare, analyse, outline, assess and discuss.
Your teacher may be able to provide a copy of of these definitions. If not you can get a copy from the Board of Studies web site (but it will be a test of your computer skills!). Go to www.boardofstudies.nsw.edu.au/syllabus_hsc/glossary_keywords.html. Find Chemistry then either open or download science_support6.doc (a Microsoft Word document), go to page 8 and print it. You could open or download science_support.PDF (but that is a very big file and you need Acrobat Reader to open it: see above) and go to page 165 and print it.4. Compulsory experiments
The exam tests your knowledge of experiments you were required to perform, in this case measuring a heat of combustion (question 17) and preparation of an ester (Question 22). In addition for each option there is a question about an experiment you were required to perform. You can expect this testing of these and other experiments to continue in subsequent exam papers. You need to know the details of the procedure you followed, why you did things the way you did and how the experiment could be improved.
This phrase was used in questions in three of the options. It was first introduced (without definition) on page 17 of the syllabus document. Presumably it refers to what most standard textbooks refer to as 'accuracy and precision' but which would perhaps better be termed 'accuracy and reproducibility'.
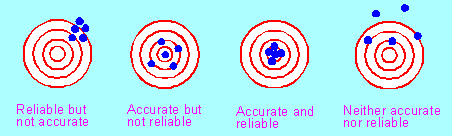
Accuracy means the closeness the measured value is to the true value. Precision (or reliability) means the reproducibility of a measurement – how easy it is to get the same answer from repeat measurements. An experiment may give reproducible results but that does not mean that they are necessarily accurate. The meanings are illustrated in the following diagrams in which shooters fire five shots at a target aiming to hit the bullseye (the centre circle).

Reliability (reproducibility) depends upon how carefully we can control all the variables that affect the results of an experiment such as temperature, heat losses to surroundings, material losses in transfers. It also depend upon how accurately we can make individual measurements; for example if we have to determine a small mass by the difference between two large masses such as 167.3 – 165.1 g then the determined mass, 2.2 g is accurate to only ± 0.2 g so repeat measurements will differ considerably.
Accuracy depends upon the design of the experiment; for example, if a volumetric analysis is planned so that the final titration is only about 3 mL then the experiment will not be accurate because we can only determine that volume to ± 0.2 or ± 0.3 mL (about a 10% error). If we redesign the experiment so that the final titration is about 30 mL, the actual error will still be the same ± 0.2 or ± 0.3 mL but that is now only a 1% error.6. Closeness to syllabus wording
Several questions such as 16, 19, 24 and 25 involve wording that is very similar to statements in the syllabus document. It is therefore essential that you are able to describe these things (and similar ones from other parts of the syllabus). In other words the new HSC requires that you learn off a lot more facts than had previously been required and not just rely on understanding basic principles and being able to apply them – in the view of many of us, not a change for the better!
You need a copy of the exam paper to make sense of what follows. If you do not already have one, you can get a copy from the Board of Studies web site, www.boardofstudies.nsw.edu.au/hsc_exams/hsc2001exams/index.html (Click on it to go there; find Chemistry in the alphabetical list, click on the 'yes' in the exams column (middle one): after the file loads print it out in your normal way.)
Section I part A (Multiple Choice questions)
| Question & Answer | Comment (and
relevant page in CCHSC if appropriate) |
|
| 1. | D | Simple recall of fact. (page 12) |
| 2. | A | Again simple factual recall. (page 23) |
| 3. | C | You need to recognise the equation that conforms to the definition of catalytic cracking – breaking a big hydrocarbon molecule into smaller ones. Catalytic cracking occurs at elevated temperatures in the gas phase so the use of liquid states in this equation could lead to confusion. (pages 5-6) |
| 4. | B | What should have been a simple recognition of a structure was made difficult by the very poor structures given (no attempt at showing three dimensions). (page 22-3, also page 450) |
| 5. | A | Another fact you need to know (pages 116-7 and 160). NO2 contributes to the low pH of polluted rain water (acid rain, pages 116-8) but is not involved in unpolluted rain water. |
| 6. | C | A simple problem. Note that in the gaps between the thick lines the colour will be changing from the left colour to the right one (that is, mixed colours in those regions) so in the gap for phenolphthalein solutions will have some colour – definitely not colourless. So yellow in methyl orange means pH > 4.5; blue in bromothymol blue means pH > 7.5; colourless in phenolphthalein means pH < 8.5. C is the only answer that fits all three requirements. (pages 128-9) |
| 7. | A | Another pH problem. The information given shows that an alkali changes the red solution to purple while acid leaves it unchanged at red. The only alkaline solution in the list is A. (pages 99-101) |
| 8. | B | There are no traps in this simple calculation. Just recognise that the molar volume of a gas is required and it is given on the data sheet. The equation shows that the 0.25 mol of S (8.00/32.07) used will produce 0.25 mole SO2. (pages 119-21) (On the basis of the data and the implied accuracy, the answer should be 6.10 L but lets not quibble!) |
| 9. | D | Something you need to know – or rather know that Le Chatelier's principle does not tell you anything about the other three possibilities. (page 109) |
| 10. | C | The only reaction involving SO42– is the second one and it is not an equilibrium reaction (it goes to completion – single-direction arrow) so adding extra sulfate will have no effect upon it. The trap was to regard this reaction as an equilibrium one and apply Le Chatelier's principle and so choose D. (page 109) |
| 11. | B | Again a fact you have to know. (page 279) |
| 12. | D | Another test of factual knowledge, but it was important to read the choices carefully, because on a quick reading A and B could appear correct: in A atomic absorption does measure chemical pollutants in water supplies but not all chemical pollutants; in B atomic absorption is a method of determining the concentration of metal ions in water supplies but it was not the first method. (pages 218-21 and 271) |
| 13. | A | From your laboratory work you should know the common causes of error in a gravimetric analysis such as this. Not drying the precipitate completely is a common error: the precipitate is too heavy because it still contains a lot of water. As long as you add sufficient precipitating solution (barium nitrate in this case), it does not matter how much excess you add (within reason). The reaction involved here is an ionic one (a precipitation reaction) and these are generally fast so you do not need to wait long for the reaction to go to completion (though you do need to stir for several minutes to ensure that all the barium sulfate coagulates into an easily filterable precipitate. (pages 211-2) |
| 14. | B | A test of your ability to interpret diagrams. C will not filter the water at all: it passes straight through the gap between the two blocks of filter membrane; in D the inlet tube will quickly get blocked up with contaminant. Both A and B will work but B offers the larger surface area of membrane so will filter the incoming water more quickly. (pages 281-2) |
| 15. | C | A test of your knowledge of solubilities of cations; you need to know that the chloride, sulfate and carbonate of lead are all insoluble. (inside back cover of CCHSC or page 215 of CCPC) |